Abstract
Parameters determining ionic transport numbers in transdermal iontophoresis have been characterized. The transport number of an ion (its ability to carry charge) is key to its iontophoretic delivery or extraction across the skin. Using small inorganic ions, the roles of molar fraction and mobility of the co- and counterions present have been demonstrated. A direct, constant current was applied across mammalian skin in vitro. Cations were anodally delivered from either simple M+Cl− solutions (single-ion case, M+ = sodium, lithium, ammonium, potassium), or binary and quaternary mixtures thereof. Transport numbers were deduced from ion fluxes. In the single-ion case, maximum cationic fluxes directly related to the corresponding ionic aqueous mobilities were found. Addition of co-ions decreased the transport numbers of all cations relative to the single-ion case, the degree of effect depending upon the molar fraction and mobility of the species involved. With chloride as the principal counterion competing to carry current across the skin (the in vivo situation), a maximum limit on the single or collective cation transport number was 0.6–0.8. Overall, these results demonstrate how current flowing across the skin during transdermal iontophoresis is distributed between competing ions, and establish simple rules with which to optimize transdermal iontophoretic transport.
INTRODUCTION
The stratum corneum, which is the outermost skin layer, constitutes a formidable barrier both to the loss of tissue water and to the entry of xenobiotics into the body. Overcoming this barrier constitutes the major challenge in transdermal drug delivery. Iontophoresis uses a mild electric current to efficiently enhance and control the flux of molecules across the skin (1–4), features which have led to the recent commercialization of a device to deliver lidocaine (LidoSite topical system, Vyteris, Fairlawn, NJ) and to the imminent approval of another containing fentanyl (E-TRANS, Alza, Mountain View, CA). Furthermore, the symmetry of iontophoresis renders possible the noninvasive extraction of substances from the subdermal interstitial fluids. Thus, iontophoresis has also found applications in the field of clinical chemistry (via, for example, the Glucowatch G2 Biographer (Cygnus, Sunnyvale, CA)) and therapeutic drug monitoring (5–8).
The transdermal fluxes of ions triggered by constant-current iontophoresis are predicted by Faraday's law (9,10):
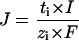 |
(1) |
where Ji, ti, and zi are the flux (mol/s), transport number, and valence of ion i, I is the current applied (in Amperes), and F is Faraday's constant (Coulombs/mol). The transport number (ti) is the fraction of the total current transported by a specific ion and expresses its efficiency as a charge carrier, ti = Ii/I. It follows that, once the transport number is known, the feasibility of its iontophoretic delivery or extraction should be easily predictable.
The sum of the transport numbers of all the ionic species present during iontophoresis must add up to 1 (Σti = 1), illustrating the competitive nature of electrotransport (11–13). Thus, the iontophoretic flux of the ion of interest depends on the ionic composition of the solutions contacting the outer and inner surfaces of the skin's barrier, and it has been suggested that the transport number may be estimated from Eq. 2, which describes the efficiency of the target ion (i) to carry current within a well stirred solution relative to the total number (j) of ions present (10,14):
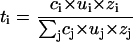 |
(2) |
where c, u, and z represent the concentration, mobility, and charge, respectively. Eq. 2 applies to a microporous membrane when the solutions bathing both sides are identical; otherwise, a more complex combination of the external concentrations must be constructed (14). It follows that the transport efficiency of an ionic drug will depend on 1), its physicochemical properties, which determine mobility and concentration, and 2), the corresponding characteristics and concentration of the co-ions and counterions present. Unfortunately, the practical utility of Eq. 2 is challenging because its rigorous application requires knowledge of ionic mobilities and concentrations inside the skin. As a result, transport numbers are usually determined experimentally from in vitro experiments and calculations based upon Eq. 1 (15,16); these results, however, are only valid for the specific set of experimental conditions in which they are obtained.
Nevertheless, Eq. 2 has been used to predict the iontophoretic transport of two competing cations (10), and to demonstrate that their molar fractions in the anode solution are critical (an observation confirmed later for lidocaine transport in the presence of varying concentrations of Na+ (16)). However, the quantitative application of the model is limited, because it applies only to neutral and homogenous membranes and requires the introduction of empirical factors to correct for ionic interactions.
Transdermal iontophoretic transport has also been described using Nernst-Planck electrodiffusion theory. In particular, the “electroneutrality” approximation has been applied to the case of a 1:1 electrolyte transporting through an uncharged membrane (17). In this case, the transport number of a monovalent species M+, present as its chloride salt, when normal saline alone fills the subdermal compartment, is given by
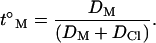 |
(3) |
DM and DCl are the diffusion coefficients of M+ and Cl− in the membrane. The efficiency of electrotransport is largely determined, therefore, by the ratio of the diffusivities of the target ion and the counterion; the concentration of M+ does not appear in the equation as it is the only cation in the anodal solution. On the contrary, in the presence of competing cations, the transport number of M+ becomes a strong function of its concentration. The validity of this approach has been demonstrated qualitatively for constant-current iontophoresis (18–20); again, however, its use as a predictive tool is limited because absolute values of DM and DCl are unknown.
Another approach has involved an attempt to relate iontophoretic flux to different physicochemical properties, such as specific conductivity (21,22). However, the latter is a function of both the concentration and mobility of all the ions present in the electrode chamber (both anions and cations) and this limits its value as a predictor of the transport of a single ion. Although it has been suggested that this problem may be circumvented by the determination of ionic mobilities from equivalent conductance at infinite dilution (19), the hypothesis has not yet been tested.
In summary, the available models are qualitatively useful but restricted in practice to the transport of two competing co-ions through a homogenous and uncharged membrane. However, it is known that the skin, under normal circumstances, is a negatively charged, cation-permselective membrane. Under the influence of an electric field, therefore, a convective, electroosmotic flow proceeds in the anode-to-cathode direction (23,24), supplementing cationic transport during iontophoresis and allowing the enhanced transport of neutral polar substances such as glucose or mannitol (25,26). Furthermore, iontophoretic transport across the skin takes place via both intercellular and appendageal routes (27). Describing and integrating this additional complexity into a single model, and then demonstrating its validity, are challenging objectives yet to be achieved, such that it is not possible at this time to predict the complex relations that determine transport numbers in a multi-ionic environment. Still, it is exactly this challenge with which one is confronted in the development of pharmaceutical formulations that typically require, in addition to the active species, excipients such as buffers, preservatives, and other components. Although charged additives will clearly decrease drug transport efficiency, the degree to which the transport is reduced is not easily predicted. In reverse iontophoresis, the presence of endogenous ions limits the extraction efficiency of the analyte of interest. In addition, for the approach to be useful, the analyte transport number must depend directly on its concentration in the interstitial fluid, in which high concentrations of sodium and chloride and a complex mixture of other endogenous ions are present. Nevertheless, this complexity has been taken advantage of in the development of a noninvasive procedure to calibrate reverse iontophoretic devices: using a so-called “internal standard”, the extraction of the analyte of interest is normalized to that of a second substance, the subdermal concentration of which is known and fixed (26,28). This method has been used to noninvasively predict lithiemia in bipolar patients, with sodium acting as the internal standard (29). For this strategy to work, the internal standard must be extracted at a constant iontophoretic flux (i.e., its transport number must be invariable). Although it is known that the systemic level of Na+ varies only between quite tight limits, the applicability of the internal-standard hypothesis requires validation that the reverse iontophoretic flux of Na+ is not influenced significantly by variations in the levels of other ions in the interstitial fluid milieu.
Thus, the aims of this work are to elucidate the criteria that determine the iontophoretic transport of ions across the skin, and optimize the delivery/extraction of target species. In particular, the manner in which the transport of charge is distributed among competing co-ions has been investigated. For the moment, the skin itself is treated as a “black box”, without imposing specific attributes to either the membrane or the pathway. Instead, the iontophoretic transport of a series of cations has been systematically evaluated. In a first step, the electromigration of monovalent inorganic cations is studied and their transport efficiency as single-ion carriers in the presence of chloride counterions is characterized. Next, co-ion competition is investigated using binary cation mixtures. Finally, quaternary mixtures are considered and the distribution of charge-carrying responsibility in complex and changing ionic environments is revealed.
MATERIALS AND METHODS
Materials
Sodium chloride, lithium chloride, ammonium chloride, and magnesium chloride were obtained from Fluka (St. Quentin Fallavier, France). Potassium chloride was purchased from Sigma-Aldrich (St. Quentin Fallavier, France). Deionized water was used for preparing all the solutions (resistivity >18 MOhm/cm2).
Skin preparation
Porcine ears were obtained fresh from the local slaughterhouse (Annecy, France) and cleaned under cold running water. The tissue was dermatomed to a thickness of 750 μm (Zimmer Air Dermatome, Dover, Ohio) and cut into small squares (9 cm2) which were wrapped individually in Parafilm and maintained at −20°C for no longer than 1 month before use. Each experiment used skin from at least four different pigs.
Iontophoresis
The skin was clamped between the two halves of side-by-side diffusion cells (transport area 0.78 cm2) with the stratum corneum facing the anodal chamber. Three to nine replicates were performed for each condition. Both the donor and the receptor chambers were filled with deionized water during two equilibrating periods of 30 min. Subsequently, the cathodal compartment was filled with 3 mL of a 5-mM MgCl2 solution. This solution was chosen to provide a source of chloride which is the principal endogenous counterion limiting iontophoretic cation delivery. MgCl2 was chosen rather than a physiological concentration of NaCl because the latter would have made the electrotransport of Na+ from the anode very difficult to measure. The concentration of MgCl2 was only 5 mM to minimize interference of the Mg2+ peak with those of the other cations in the ion chromatogram. The anodal chamber was filled (3 mL) with the respective chloride salt(s) of the cation(s) tested in each experiment (see below). Chloride salts were used because they provide the chloride ions required for the anodal electrochemistry. Constant direct current (0.4 mA) was applied for 6 h via Ag/AgCl electrodes connected to a power supply (Kepco, MB Electronique, Bron, France). The solutions in the electrode chambers were magnetically stirred throughout the experiment.
Experimental design
Single ions
The anodal solution was a 100-mM chloride salt of one of the four cations tested (Na+, 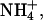 K+, and Li+). These experiments examined the “single-carrier” or “single-ion” situation, i.e., they determined the capability of each cation to compete for charge transport against chloride counterions. The composition of both electrode chambers (Table 1) was kept constant throughout the experiment (6 h). The entire anodal and cathodal solutions were sampled every hour and the electrode chambers refilled with fresh solutions. This procedure avoided artifacts due to depletion of the ionic content.
K+, and Li+). These experiments examined the “single-carrier” or “single-ion” situation, i.e., they determined the capability of each cation to compete for charge transport against chloride counterions. The composition of both electrode chambers (Table 1) was kept constant throughout the experiment (6 h). The entire anodal and cathodal solutions were sampled every hour and the electrode chambers refilled with fresh solutions. This procedure avoided artifacts due to depletion of the ionic content.
TABLE 1.
Composition of the anodal solutions for the single-ion and binary combination experiments
| Experiment code | Carrier ion(s) (mM) | |
|---|---|---|
| Single-ion S.C+ (n) | ||
| S.Li+ | (6) | Li+ (100) |
| S.Na+ | (9) | Na+ (100) |
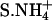 |
(3) | 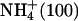 |
| S.K+ | (6) | K+ (100) |
| Binary combination B.C+ (mM) (n) | ||
| B.Li+ (25) | (7) | C+ (25) |
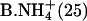 |
(6) | Na+ (100) |
| B.K+ (25) | (6) | – |
| B.Li+ (50) | (7) | C+ (50) |
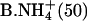 |
(6) | Na+ (100) |
| B.K+ (50) | (5) | – |
| B.Li+ (100) | (7) | C+ (100) |
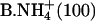 |
(7) | Na+ (100) |
| B.K+ (100) | (6) | – |
| B.Li+ (20) | (5) | C+ (20) |
| B.K+ (20) | (3) | Na+ (20) |
In all experiments, the cathodal solution was 5 mM MgCl2 (i.e., 10 mM Cl−).
Binary combinations
These experiments (Table 1) tested the ability of K+, 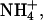 and Li+ to compete with Na+. The first series of studies (B.C+25, B.C+50, and B.C+100) investigated a fixed level of sodium (100 mM) competing with progressively higher concentrations of each co-ion (25, 50, and 100 mM). The sodium molar fraction (
and Li+ to compete with Na+. The first series of studies (B.C+25, B.C+50, and B.C+100) investigated a fixed level of sodium (100 mM) competing with progressively higher concentrations of each co-ion (25, 50, and 100 mM). The sodium molar fraction (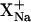 ) was decreased therefore from 0.8 to 0.5 in these measurements. In a second set of experiments (B.C+20 and B.C+100), on the other hand, a constant molar ratio of the two was considered (either 100:100 mM or 20:20 mM). The composition of both electrode chambers was again kept constant throughout the experiments. The entire anodal and cathodal solutions were sampled every hour and the electrode chambers refilled with fresh solutions.
) was decreased therefore from 0.8 to 0.5 in these measurements. In a second set of experiments (B.C+20 and B.C+100), on the other hand, a constant molar ratio of the two was considered (either 100:100 mM or 20:20 mM). The composition of both electrode chambers was again kept constant throughout the experiments. The entire anodal and cathodal solutions were sampled every hour and the electrode chambers refilled with fresh solutions.
Multiple-ion combinations
The aim of these experiments (Table 2) was to study how the charge flowing across the skin during iontophoresis is distributed in more complex situations. Experiment M.1 employed an anodal formulation containing 50 mM of each cation (Li+, Na+, 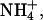 and K+) chloride. The remaining experiments evaluated how the cations' transport numbers were modified by changes in the ionic composition of the donor formulation. Iontophoresis was initiated with one of the anodal compositions in Table 2 and samples were taken hourly for 3 h. The donor solution was then replaced as indicated in Table 2. Subsequently, samples were taken every half-hour during a second 3-h period of iontophoresis. Experiments M.2 and M.3 examined the impact of a sharp decrease in sodium concentration on the fluxes of the competing co-ions. Finally, experiment M.4 considered the effect of simultaneously increasing the concentration of ammonium and potassium.
and K+) chloride. The remaining experiments evaluated how the cations' transport numbers were modified by changes in the ionic composition of the donor formulation. Iontophoresis was initiated with one of the anodal compositions in Table 2 and samples were taken hourly for 3 h. The donor solution was then replaced as indicated in Table 2. Subsequently, samples were taken every half-hour during a second 3-h period of iontophoresis. Experiments M.2 and M.3 examined the impact of a sharp decrease in sodium concentration on the fluxes of the competing co-ions. Finally, experiment M.4 considered the effect of simultaneously increasing the concentration of ammonium and potassium.
TABLE 2.
Composition of the anodal solutions for the multiple-ion combination experiments
| Experiment code (n) | Time (min) | Carrier ions (mM) |
|---|---|---|
| M.1 (6) | 0–300 | Li+ (50), K+ (50) |
 Na+ (50) Na+ (50) | ||
| M.2 (6) | 0–180 | Li+ (50), K+ (25) |
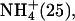 Na+ (100) Na+ (100) | ||
| 180–360 | Li+ (50), K+ (25) | |
 Na+ (25) Na+ (25) | ||
| M.3 (5) | 0–180 | Li+ (25), K+ (50) |
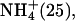 Na+ (100) Na+ (100) | ||
| 180–360 | Li+ (25), K+ (50) | |
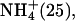 Na+ (25) Na+ (25) | ||
| M.4 (6) | 0–180 | Li+ (25), K+ (50) |
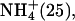 Na+ (25) Na+ (25) | ||
| 180–360 | Li+ (25), K+ (100) | |
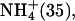 Na+ (25) Na+ (25) |
In all experiments, the cathodal solution was 5 mM MgCl2 (i.e., 10 mM Cl−).
Sample analysis
Lithium, sodium, potassium, and ammonium ions were assayed by ion chromatography on a Dionex ion chromatograph 600 system (Dionex, Sunnyvale, CA) equipped with a gradient pump, a thermal compartment, and an electrochemical detector. Separation was achieved with a cation-exchange column preceded by a guard column through which a 25-mM methanesulfonic acid mobile phase was perfused. Quantification was performed in the suppressed conductivity mode; the electric current applied to the suppressor was 88 mA. A calibration was carried out with at least five standards for Li+, Na+, 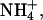 and K+.
and K+.
Data analysis
Transport numbers were determined for each sampling period via Eq. 1. The values reported correspond to the mean ± SD of 3–9 replicates. Statistical analysis used Prism 4 (GraphPad Software, San Diego, CA). One- and two-way ANOVAs followed by the corresponding Tukey and Bonferroni tests were used to analyze the data from single-ion and binary-cation experiments. The level of statistical significance was fixed at p < 0.05. All linear regression procedures were followed by the corresponding ANOVAs to test the significance of the regression.
RESULTS AND DISCUSSION
As described above, no buffer was used in any of the experiments in this study so as to avoid the presence of additional competing species. The pH of the subdermal receptor solution was 6, whereas that of the donor varied between 5 and 6, depending on the ionic composition. The dominant mechanism of iontophoretic transport for the small inorganic cations examined here is electromigration which is generally less sensitive to pH than electroosmosis (30). Indeed, when the effect of pH was assessed using two 10-mM NaCl donor solutions, it was found that the Na+ transport numbers at pH 5 and 7 were 0.59 ± 0.01 and 0.64 ± 0.03, respectively. Because all experiments reported here employed the same cathodal electrolyte (5 mM MgCl2, pH ∼6), the small pH variations in the donor solutions were considered insufficient to significantly modify cation transport from the anode across the skin.
Single ions
The single carrier condition is optimal for iontophoretic drug delivery. Above all, this situation ensures maximum drug flux (i.e., maximum transport number) since the competition to carry the current is limited to endogenous counterions beneath the skin. Second, Eq. 3 (17) predicts that the ion's transport number under these conditions (t°i) is independent of applied concentration. From a practical point of view, this allows maximum flux to be achieved at low “loading”, a convenient feature for the delivery of expensive drugs such as peptides. The validity of this prediction has been demonstrated in vitro for lidocaine (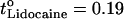 ), hydromorphone (
), hydromorphone ( ), and for ropinirole both in vitro (
), and for ropinirole both in vitro (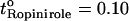 ) and in vivo (
) and in vivo ( ) (16,18–20). Because t°i determines the feasibility of drug delivery by iontophoresis, it would be extremely useful to predict this parameter from simple physicochemical properties. In this first series of experiments; the t°i of lithium, sodium, potassium, and ammonium were determined with chloride as the competing counterion. The results are shown in Table 3 and are compared to the corresponding aqueous transport numbers (31). The latter clearly reflect the mobility of each cation relative to that of chloride (
) (16,18–20). Because t°i determines the feasibility of drug delivery by iontophoresis, it would be extremely useful to predict this parameter from simple physicochemical properties. In this first series of experiments; the t°i of lithium, sodium, potassium, and ammonium were determined with chloride as the competing counterion. The results are shown in Table 3 and are compared to the corresponding aqueous transport numbers (31). The latter clearly reflect the mobility of each cation relative to that of chloride (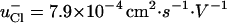 ). As expected, the aqueous mobilities of the cations are related to hydrodynamic radius (as opposed to atomic or molecular weight, or to ionic radius (32)). Small ions, of course, are extensively solvated and, hence, the effective size of Li+, for example, is greater than those of K+ and
). As expected, the aqueous mobilities of the cations are related to hydrodynamic radius (as opposed to atomic or molecular weight, or to ionic radius (32)). Small ions, of course, are extensively solvated and, hence, the effective size of Li+, for example, is greater than those of K+ and 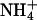 .
.
TABLE 3.
Cation transport numbers in water and in the skin during transdermal iontophoresis
| Water
|
Skin
|
||||||
|---|---|---|---|---|---|---|---|
| Cation | Atomic weight | Ionic radius* (Å) | Hydrodynamic radius† (Å) | Mobility* (10−4 cm2·s−1·V−1) |
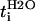 ‡ ‡
|
t°i, SC+ ± SD (Expt. S.C+) | t°i, M1 ± SD (Expt. M.1) |
| Li+ | 6 | 0.060 | 1.73 | 4.01 | 0.33 | 0.54 ± 0.06§(a) | 0.13 ± 0.01§(b,c) |
| Na+ | 23 | 0.095 | 1.67 | 5.19 | 0.39 | 0.59 ± 0.06¶ | 0.18 ± 0.01§(b) |
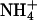 |
18 | 0.133 | 1.14 | 7.60 | 0.49 | 0.77 ± 0.19§¶(a) | 0.22 ± 0.02§(b) |
| K+ | 39 | 0.143 | 1.14 | 7.62 | 0.49 | 0.70 ± 0.15 | 0.22 ± 0.01§(c) |
Values were deduced from the results of single-carrier and quaternary combination donor formulations.
Ionic radius and mobility data were taken from Atkins (32).
Hydrodynamic radius calculated from ionic mobility (32).
The transport numbers in water were taken from Falk (31).
(a,b,c)Values significantly different (one-way ANOVA, Tukey test, p < 0.01).
Values significantly different (one-way ANOVA, Tukey test, p < 0.05).
The sodium and potassium transport numbers are similar to those reported previously (∼0.5–0.6) for human and pig skin (15,33,34); the differences are attributable to the different experimental conditions (buffers, pH) and methods employed. Cationic transport numbers across skin are significantly greater than those in aqueous solution because of the membrane's net negative charge (34). From a practical point of view, this means that iontophoresis more efficiently delivers cationic drugs, a deduction well supported by experimental observation (35,36).
The results of these experiments define an upper limit for drug delivery by iontophoresis; that is, no drug can do better than these small, inorganic cations when competing with endogenous chloride (concentration >100 mM) transporting current in the opposite direction. Lithium, therefore, would be the best drug candidate for iontophoretic delivery (it is presently administered orally to treat bipolar disorder) and its transport number in Table 3 may be considered as an upper limit for the charge-carrying efficiency of cationic drugs. All other drugs are larger and less mobile than lithium, and will therefore transport <54% of the charge when confronted with the physiological concentration of subdermal chloride.
It is worth noting that the relative order of cation transport numbers in water and through the skin is similar: 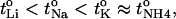 with the values for lithium being significantly less than those of potassium and ammonium (Table 3). As discussed previously, the
with the values for lithium being significantly less than those of potassium and ammonium (Table 3). As discussed previously, the 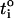 has been suggested to be a function of the diffusivities of the cation and its counterion (17). Equally, the Einstein relationship predicts a direct proportionality between diffusivity and mobility (D = uiRT/ziF) (32). However, it is difficult to measure ionic diffusivities or mobilities within the skin (37), begging the question, therefore, as to whether values measured in water may be used to predict the corresponding transport number in skin for the single carrier situation. Although the results in Table 3 are consistent with this idea, and the much lower mobilities of ropinirole and lidocaine (1.6 × 10−4 cm2·s-1·V-1 and 1.5 × 10−4 cm2·s−1·V−1, respectively) correlate with their significantly smaller transport numbers, the hypothesis requires a larger data set before it can be confirmed or refuted.
has been suggested to be a function of the diffusivities of the cation and its counterion (17). Equally, the Einstein relationship predicts a direct proportionality between diffusivity and mobility (D = uiRT/ziF) (32). However, it is difficult to measure ionic diffusivities or mobilities within the skin (37), begging the question, therefore, as to whether values measured in water may be used to predict the corresponding transport number in skin for the single carrier situation. Although the results in Table 3 are consistent with this idea, and the much lower mobilities of ropinirole and lidocaine (1.6 × 10−4 cm2·s-1·V-1 and 1.5 × 10−4 cm2·s−1·V−1, respectively) correlate with their significantly smaller transport numbers, the hypothesis requires a larger data set before it can be confirmed or refuted.
Binary combinations
The goal of these experiments was to study co-ion competition in the simplest system possible: i.e., two cations (one of which was always sodium) competing against subdermal chloride. Sodium ion competition with other cations is relevant because: 1), sodium (as saline) is a very common additive to drug formulations (as a buffer component, stabilizing agent, etc.), and 2), in reverse iontophoresis, the use of sodium as an internal standard requires that its iontophoretic flux remains constant despite variation in the ionic composition of the interstitial fluid.
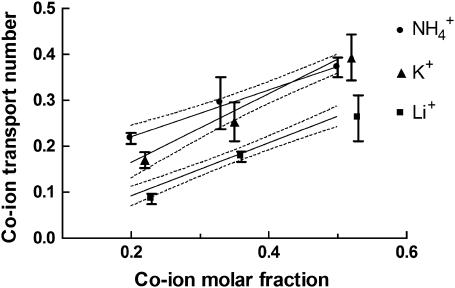
According to Eq. 2, the mobility and concentration inside the skin are the principal factors that determine the transport number of monovalent ions. For hydromorphone and lidocaine (16,18), molar fraction is a better predictor of iontophoretic flux than the nominal concentration, as it reflects the drug level relative to the total concentration of cations present. On the other hand, the iontophoretic flux of ropinirole revealed more complicated behavior when its concentration and that of competing sodium ions were changed in parallel (19). One series of experiments performed with monovalent inorganic cations (Table 1: B.C+25, B.C+50, and B.C+100) involved a constant concentration of sodium ions competing with a progressively higher level of a second cation (Li+, K+, or 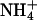 ). As a result, the sodium molar fraction decreased from 0.8 to 0.5, whereas that of the competitor increased from 0.2 to 0.5. In experiments B.C+20 and B.C+100 the two competing cations were introduced at the same millimolar concentrations, 20:20 and 100:100, such that their molar fractions were kept constant. The results (Table 4 and Figs. 1 and 2) were as follows:
). As a result, the sodium molar fraction decreased from 0.8 to 0.5, whereas that of the competitor increased from 0.2 to 0.5. In experiments B.C+20 and B.C+100 the two competing cations were introduced at the same millimolar concentrations, 20:20 and 100:100, such that their molar fractions were kept constant. The results (Table 4 and Figs. 1 and 2) were as follows:
Both molar fraction and the identity of the competitor ion were significant factors determining the transport number (two-way ANOVA, p < 0.0001). The transport number of lithium was significantly smaller (p < 0.001) than those of potassium and ammonium under equivalent conditions, presumably due to lithium's lower mobility. Practically speaking, this means that the introduction of sodium ions into an iontophoretic formulation of a cationic drug will have an impact dependent upon the drug's mobility; that is, the drug's transport number will be reduced, and the decrease will be more important for the least mobile drugs. Thus, whenever practical, formulation additives should be selected from low-mobility, and ideally uncharged, species that are introduced at the lowest suitable concentration possible.
The experiments B.C+25, B.C+50, and B.C+100 (Table 4 and Fig. 1) show that the transport numbers of Li+, K+, and
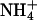 increased with concentration, whether expressed in molar units or as a molar fraction. Comparison of the results from experiments B.C+20 and B.C+100 (Table 4) showed that molar fraction is a better predictor of transport number than molarity, because it expresses the level of the cation of interest with respect to the total ionic background. The results reported here agree with those obtained previously for lidocaine and hydromorphone (16,18) and, interestingly, a similar relationship can be deduced from data published in 1929 from experiments examining the competition between sodium and potassium ions in aqueous solutions (31).
increased with concentration, whether expressed in molar units or as a molar fraction. Comparison of the results from experiments B.C+20 and B.C+100 (Table 4) showed that molar fraction is a better predictor of transport number than molarity, because it expresses the level of the cation of interest with respect to the total ionic background. The results reported here agree with those obtained previously for lidocaine and hydromorphone (16,18) and, interestingly, a similar relationship can be deduced from data published in 1929 from experiments examining the competition between sodium and potassium ions in aqueous solutions (31).Another relevant question is the manner in which the transport number of a drug increases with concentration in the formulation. Clearly, as the sum of transport numbers of the ions must add up to 1, there is a maximum limit for the flux (i.e., the ti) of each species. Fig. 1 indicates linear relationships between the transport numbers of Li+, K+, and
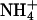 and their respective molar fractions. The ion's transport number in the single-ion situation can be predicted from these regression equations by setting the molar fraction equal to 1. The values obtained for Li+, K+, and
and their respective molar fractions. The ion's transport number in the single-ion situation can be predicted from these regression equations by setting the molar fraction equal to 1. The values obtained for Li+, K+, and 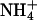 are 0.56, 0.76, and 0.63, respectively, in excellent agreement with those measured experimentally in the single-ion experiments (Table 3). Finally, it is noted that the gradient of the regression in Fig. 1 for lithium is significantly lower (p < 0.04) than that for potassium, a finding due, perhaps, to the different mobilities of these ions. Similarly, for lidocaine, under comparable experimental conditions, the gradient was 0.18 (16). These results suggest that the transport numbers of more mobile drugs across the skin increase more quickly with molar fraction (that is, in accord with Eq. 3, it is not simply the relative concentrations of the ions that are important, but also their mobilities: smaller, more mobile ions will “grab” a larger fraction of the charge). The principle has also been demonstrated in aqueous solutions where regression slopes for Na+ and K+ (31) were 0.35 and 0.47, respectively. Nevertheless, further research with more compounds is required to confirm this hypothesis.
are 0.56, 0.76, and 0.63, respectively, in excellent agreement with those measured experimentally in the single-ion experiments (Table 3). Finally, it is noted that the gradient of the regression in Fig. 1 for lithium is significantly lower (p < 0.04) than that for potassium, a finding due, perhaps, to the different mobilities of these ions. Similarly, for lidocaine, under comparable experimental conditions, the gradient was 0.18 (16). These results suggest that the transport numbers of more mobile drugs across the skin increase more quickly with molar fraction (that is, in accord with Eq. 3, it is not simply the relative concentrations of the ions that are important, but also their mobilities: smaller, more mobile ions will “grab” a larger fraction of the charge). The principle has also been demonstrated in aqueous solutions where regression slopes for Na+ and K+ (31) were 0.35 and 0.47, respectively. Nevertheless, further research with more compounds is required to confirm this hypothesis.-
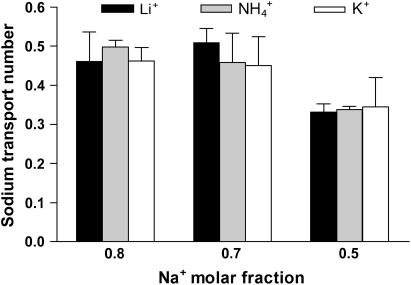
The transport number of Na+ did not decrease significantly until its molar fraction was reduced to 0.5; the decrease appeared to be independent of the nature of the competitor (Fig. 2 and Table 4), a result confirmed by a two-way ANOVA on the effect of sodium concentration and competitor ion on the sodium transport number. The identity of the competitor was not important, whereas the sodium transport number showed a significant reduction only at the lowest molar fraction considered (p < 0.001).
These results are relevant to the use of sodium as an internal standard in reverse iontophoresis. Sodium concentration in plasma varies from 135 to 145 mM; other cations present at the millimolar level are potassium (3.4–4.8 mM), calcium (2.1–2.6 mM, 50–60% ionized and partially bound to proteins), and magnesium (0.68–0.88) (38). Other cations are found at the micro or nanomolar level. Thus, under normal physiological circumstances, the sodium molar fraction in blood should be ≥0.94. An increase in potassium to 10 mM (the highest value reported for a severe hyperkalemia (38)) would only decrease the sodium molar fraction to 0.91. Therefore, the results reported are consistent with previous in vitro and in vivo observations on the constancy of sodium iontophoretic flux and its role as an internal standard (26,29,39). In fact sodium molar fraction would have to decrease to levels incompatible with life before the iontophoretic flux of this ion is significantly modified. That is, even though Na+ mobility is less than those of K+ and
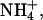 the impact of this difference is too small to be observed experimentally until the concentrations of the ions become (nonphysiologically) similar.
the impact of this difference is too small to be observed experimentally until the concentrations of the ions become (nonphysiologically) similar. A final observation is that the sum of the cation transport numbers is quite constant (Table 4) despite the large variations studied in the total concentration of these species. Experiments B.C+20 and B.C+100 are particular examples: a fivefold increase in cation concentration (40–200 mM) had a minimal effect on the sum of the cation transport numbers. Interestingly, in classic experiments with binary donors in water, the maximum sum of Na+ and K+ transport numbers was 0.4 (31). Not unexpectedly, the presence of a cation-permselective membrane, such as the skin, results in an overall cation transport number that is higher (0.5–0.8). This effect is noteworthy, as the chloride concentration employed in this work (10 mM) was only 1/4 to 1/20 of the total cation concentration. However, it must be remembered that the single-ion situation applied to chloride here and that its transport was predicted (17) to be dependent only on its diffusivity relative to that of the cations present (for example, a lower total cationic transport number is observed in the binary experiments with lithium and sodium compared to the potassium/sodium couple (p < 0.01)). Further, although the prediction was developed for a 1:1 electrolyte, the results from this work suggest that the theory applies to more complex situations; that is, whereas the donor solutions were always 1:1 electrolytes, the receiver phase contained MgCl2, which is a 1:2 electrolyte. Thus, although there is no traceable tie between the theory cited and the experimental conditions, the results obtained are nevertheless well predicted by the relatively simplistic model. From a practical point of view, these data imply that even the best combination of competing cations in an iontophoretic vehicle will not result in a total cation transport number of >0.75, setting a clear upper limit on the iontophoretic delivery efficiency of cationic drugs.
TABLE 4.
Individual and total cation transport numbers deduced from the binary cation experiments
| Experiment | tC+ | 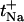 |
∑tCation |
|---|---|---|---|
| B.Li+ (25) | 0.09 ± 0.01 | 0.46 ± 0.07 | 0.55 ± 0.08 |
| B.K+ (25) | 0.17 ± 0.02 | 0.46 ± 0.03 | 0.60 ± 0.05 |
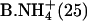 |
0.21 ± 0.01 | 0.50 ± 0.02 | 0.72 ± 0.02 |
| B.Li+ (50) | 0.18 ± 0.01 | 0.48 ± 0.05 | 0.62 ± 0.05 |
| B.K+ (50) | 0.25 ± 0.04 | 0.45 ± 0.07 | 0.70 ± 0.06 |
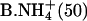 |
0.29 ± 0.06 | 0.46 ± 0.08 | 0.75 ± 0.11 |
| B.Li+ (100) | 0.26 ± 0.05 | 0.33 ± 0.02 | 0.58 ± 0.07 |
| B.K+ (100) | 0.39 ± 0.05 | 0.34 ± 0.08 | 0.71 ± 0.10 |
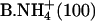 |
0.37 ± 0.02 | 0.34 ± 0.01 | 0.71 ± 0.02 |
| B.Li+ (20) | 0.21 ± 0.03 | 0.30 ± 0.03 | 0.51 ± 0.06 |
| B.K+ (20) | 0.44 ± 0.02 | 0.31 ± 0.02 | 0.75 ± 0.03 |
Values are given as mean ± SD for 3–9 experiments. Numbers in square brackets in first column signify millimolar concentration of co-ion.
FIGURE 1.
Co-ion (C+) transport number (tC+) as a function of its molar fraction in binary cation experiments (B.C+25, B.C+50, and B.C+100). Lines of linear regression are drawn through the data, which are presented as the mean ± SD (n ≥ 5). For clarity, although the upper and lower 95% confidence interval is shown for lithium, only the upper and lower 95% confidence intervals, respectively, are shown for ammonium and potassium. A two-way ANOVA indicates that both the nature and the molar fraction of C+ are determinant factors of tC+. The regression equations were tLi+ = −0.025 (±0.02) + 0.581 (±0.05) XLi, r2 = 0.86; tK+ = 0.014 (±0.03) + 0.752 (±0.08) XK, r2 = 0.85; and tNH4+ = 0.117 (±0.02) + 0.514 (±0.06) XNH4, r2 = 0.82. The three regressions were significant (p < 0.001) and the values in brackets correspond to the standard errors of the statistics.
FIGURE 2.
Sodium transport number (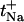 ) as a function of its molar fraction in the binary cation experiments (B.C+25, B.C+50, and B.C+100). The data are presented as the mean ± SD (n ≥ 5). A two-way ANOVA indicates that the sodium molar fraction is the only determinant factor of
) as a function of its molar fraction in the binary cation experiments (B.C+25, B.C+50, and B.C+100). The data are presented as the mean ± SD (n ≥ 5). A two-way ANOVA indicates that the sodium molar fraction is the only determinant factor of 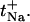
Multiple-ion combinations
It is evident that molar fraction and mobility are the key determinants of cation transport numbers in simple binary mixtures, but “real-world” iontophoretic vehicles may be more complex, incorporating both background electrolyte and buffer constituents, for example. Although the effect of sodium concentration on the iontophoretic transport of a drug has been frequently reported (11,13,16,40), the impact of a complex background electrolyte has received much less attention. Thus, the rational development of iontophoretic vehicles is complex. The last component of this study therefore examined how transport numbers are determined in complex ionic environments and how they may be modified by manipulation of the formulation.
Fixed-concentration experiments
The first experiment (Table 2, M.1) determined the distribution of charge transport among the four cations when present at the same concentration. The transport numbers were correlated with aqueous mobilities and were consistent with the values measured in the single-ion situation (Table 3). It follows that, when present at equal concentrations, the more mobile cations will transport a higher fraction of the charge, and that transport numbers determined in the single-ion situation are good predictors of the charge distribution in complex ionic environments. Once again, it is noted that the sum of the cation transport numbers is ∼0.75 (see preceding section).
Stepwise concentration-change experiments
The next experiments (Table 3, M.2 and M.3) examined the situation in which the sodium concentration in the anode formulation, initially at 100 mM, was subsequently lowered in a step change to 25 mM. The idea was to replicate, in part, the optimization of an iontophoretic vehicle. The goal was to evaluate how the initial sodium transport number was “distributed” among the other cations, and to determine which of the co-ions eventually benefited most from the reduced Na+ molar fraction. The two experiments differed in terms of the mobility of the second-most concentrated (50 mM) cation initially present: lithium (M.2) or potassium (M.3). The following observations (Table 5 and Fig. 3) are worthy of comment. Firstly, sodium and ammonium transport numbers changed in a similar fashion in both experiments, independent of the levels of potassium and lithium. The charge “captured” by sodium and ammonium is principally determined by their molar fractions in the formulation; these levels were the same in both experiments and were unaffected by the relative amounts of potassium and lithium. Second, the transport numbers of ammonium and potassium, which have equivalent mobilities in water, clearly reflected their relative concentrations in the formulation. Hence, the potassium transport number was about double that of ammonium transport numbers in M.3, whereas the two were very similar in M.2. On the other hand, lithium, which is less mobile than potassium, captured significantly less charge than potassium even when the two cations were present in equal concentrations.
TABLE 5.
Individual and total cation transport numbers deduced from the multiple-ion experiments
|
ti (mean ± SD)
|
|||
|---|---|---|---|
| M.2 | 0–180 min | 180–360 min | % change |
| Li+ | 0.11 ± 0.01 | 0.18 ± 0.01 | 64.8 ± 4.0 |
| Na+ | 0.34 ± 0.02 | 0.16 ± 0.01 | −53.9 ± 1.5 |
| K+ | 0.11 ± 0.01 | 0.19 ± 0.01 | 58.7 ± 10.1 |
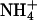 |
0.09 ± 0.01 | 0.15 ± 0.01 | 53.8 ± 7.0 |
| ∑ti+ | 0.66 ± 0.04 | 0.68 ± 0.04 | – |
|
ti (mean ± SD)
|
|||
| M.3 | 0–180 min | 180–360 min | % change |
| Li+ | 0.10 ± 0 .01 | 0.16 ± 0.01 | 56.0 ± 8.6 |
| Na+ | 0.31 ± 0.03 | 0.17 ± 0.01 | −46.9 ± 2.0 |
| K+ | 0.21 ± 0.02 | 0.32 ± 0.01 | 49.3 ± 8.0 |
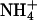 |
0.12 ± 0.02 | 0.17 ± 0.01 | 38.8 ± 11.3 |
| ∑ti+ | 0.74 ± 0.06 | 0.81 ± 0.02 | – |
|
ti (mean ± SD)
|
|||
| M.4 | 0–180 min | 180–360 min | % change |
| Li+ | 0.08 ± 0 .01 | 0.05 ± 0.01 | −30.1 ± 3.2 |
| Na+ | 0.15 ± 0.01 | 0.11 ± 0.01 | −25.6 ± 2.1 |
| K+ | 0.33 ± 0.02 | 0.42 ± 0.02 | 30.3 ± 4.1 |
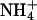 |
0.18 ± 0.02 | 0.14 ± 0.02 | −20.7 ± 5.5 |
| ∑ti+ | 0.74 ± 0.05 | 0.73 ± 0.06 | – |
FIGURE 3.
Response of cation transport numbers subsequent to a modification of the ionic composition of the anodal solution. At 3 h of iontophoresis (experiment M.3), the anodal solution was changed from LiCl (25 mM), KCl (50 mM), NH4Cl (25 mM), and NaCl (100 mM) to LiCl (25 mM), KCl (50 mM), NH4Cl (25 mM), and NaCl (25 mM). The data are presented as the mean ± SD (n ≥ 5). The lithium and ammonium transport numbers have been slightly displaced on the time axis to facilitate visualization of the data.
In experiment M.4, two cations of similar mobility are simultaneously increased, potassium from 50 to 100 mM, and ammonium from 25 to 35 mM. Transport numbers in the first step agreed with results already discussed; i.e., the roles of mobility and concentration were apparent. Lithium and sodium transport numbers in the second step decreased as expected. Surprisingly, ammonium transport numbers also decreased despite its presence at a higher concentration. This experiment underlined the critical role of molar fraction, the value of which, for 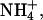 slightly decreased from 0.2 to 0.19 due to the more important increase in potassium. At a practical level, this finding suggests that an increased drug flux (i.e., a higher transport number) can be achieved by increasing the molar fraction of the drug without necessarily raising the molar concentration. Overall, these quaternary donor experiments convey the messages that 1), at equal concentration, transport numbers will align themselves as a function of the co-ions' mobilities, and 2), at equal mobilities, the transport numbers are directly proportional to molar fraction.
slightly decreased from 0.2 to 0.19 due to the more important increase in potassium. At a practical level, this finding suggests that an increased drug flux (i.e., a higher transport number) can be achieved by increasing the molar fraction of the drug without necessarily raising the molar concentration. Overall, these quaternary donor experiments convey the messages that 1), at equal concentration, transport numbers will align themselves as a function of the co-ions' mobilities, and 2), at equal mobilities, the transport numbers are directly proportional to molar fraction.
The degree to which the transport number of each cation is modified when the donor solution is altered (% change), may be calculated from
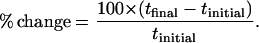 |
(4) |
For sodium, the % change in experiments M.2 and M.3 was 54 (± 1.5)% and 47 (± 2)%, respectively (Table 5). The corresponding changes in the transport numbers of Li+, K+, and 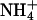 in these experiments were relatively constant (falling in the ranges of 53–65% in experiment M.2 and 39–56% in experiment M.3). In experiment M.4, the % change in tK+ was 30 (± 4)%, whereas those of the other cations fell by an amount in the range of 21–30%. It follows that the relative changes of all cations under equivalent conditions were relatively constant for a given change in the formulation.
in these experiments were relatively constant (falling in the ranges of 53–65% in experiment M.2 and 39–56% in experiment M.3). In experiment M.4, the % change in tK+ was 30 (± 4)%, whereas those of the other cations fell by an amount in the range of 21–30%. It follows that the relative changes of all cations under equivalent conditions were relatively constant for a given change in the formulation.
Finally, the sum of the cation transport numbers ranges between 0.65 and 0.85 for the six donor compositions examined over a total ionic concentration from 125 to 200 mM. That is, a total cationic transport number is conserved in a manner consistent with M.1 and binary mixture experiments. Overall, these experiments confirm that 1), cationic delivery by iontophoresis will always be restricted by endogenous chloride efflux, and 2), a maximum efficiency of 60–80% can be firmly established for positively charged drugs.
CONCLUSIONS
The transdermal iontophoresis experiments reported here support the following conclusions: 1), the single-ion situation allows maximal transport efficiency, which is correlated with aqueous mobility; 2), the presence of sodium as a competing species decreases cation transport numbers in a manner related to the relative aqueous mobilities and molar fractions in the anodal solution; 3), molar fraction, rather than absolute concentration, should be optimized for formulation purposes; 4), the reverse iontophoretic flux of sodium under physiological conditions will be constant, supporting the role of sodium as an internal standard; 5), the transport numbers of all co-ions are modified to the same extent when the ionic composition of the donor formulation is modified; and 6), despite widely different total cation concentrations at the anode, the sum of cation transport numbers maximizes at ∼0.65–0.81, and it follows that competition from subdermal chloride cannot be eliminated via changes in the iontophoretic vehicle—that is, the efficiency of cationic delivery is limited.
Acknowledgments
The financial support of Vyteris (Fair Lawn, NJ), the National Institutes of Health (EB-001420), and the Parkinson's Disease Society is gratefully acknowledged.
References
- 1.Sage, B. H., and J. E. Riviere. 1992. Model systems in iontophoresis: transport efficacy. Adv. Drug Deliv. Rev. 9:265–287. [Google Scholar]
- 2.Delgado-Charro, M. B., and R. H. Guy. 2001. Transdermal iontophoresis for controlled drug delivery and non-invasive monitoring. STP Pharma Sci. 11:403–414. [Google Scholar]
- 3.Singh, P., P. Liu, and S. M. Dinh. 2002. Facilitated transdermal delivery by iontophoresis. In Topical Absorption of Dermatological Products. R. L. Bronaugh and H. I. Maibach, editors. Marcel Dekker, New-York. 353–376.
- 4.Kalia, Y. N., A. Naik, J. Garrison, and R. H. Guy. 2004. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 56:619–658. [DOI] [PubMed] [Google Scholar]
- 5.Tierney, M. J., J. A. Tamada, R. O. Potts, R. C. Eastman, K. R. Pitzer, N. R. Ackerman, and S. Fermi. 2000. The GlucoWatch biographer: a frequent automatic and noninvasive glucose monitor. Ann. Med. 32:632–641. [DOI] [PubMed] [Google Scholar]
- 6.Tierney, M. J., J. A. Tamada, R. O. Potts, L. Jovanovic, and S. Garg, and Cygnus Research Team. 2001. Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 16:621–629. [DOI] [PubMed] [Google Scholar]
- 7.Leboulanger, B., R. H. Guy, and M. B. Delgado-Charro. 2004. Reverse iontophoresis as a noninvasive tool for lithium monitoring and pharmacokinetic profiling. Pharm. Res. 21:1214–1222. [DOI] [PubMed] [Google Scholar]
- 8.Leboulanger, B., R. H. Guy, and M. B. Delgado-Charro. 2004. Reverse iontophoresis for non-invasive transdermal monitoring. Physiol. Meas. 25:R35–R50. [DOI] [PubMed] [Google Scholar]
- 9.Burnette, R. R. 1989. Iontophoresis. In Transdermal Delivery Systems: Developmental Issues and Research Initiatives. J. Hadgraft and R. H. Guy, editors. Marcel Dekker, New York. 247–291.
- 10.Phipps, J. B., and G. Gyory. 1992. Transdermal ion migration. Adv. Drug Deliv. Rev. 9:137–176. [Google Scholar]
- 11.Clemessy, M., G. Couarraze, B. Bevan, and F. Puisieux. 1995. Mechanisms involved in iontophoretic transport of angiotensin. Pharm. Res. 12:998–1002. [DOI] [PubMed] [Google Scholar]
- 12.Thysman, S., V. Preat, and M. Roland. 1992. Factors affecting iontophoretic mobility of metoprolol. J. Pharm. Sci. 81:670–675. [DOI] [PubMed] [Google Scholar]
- 13.Lopez, R. F. V., M. V. L. B. Bentley, M. B. Delgado-Charro, and R. H. Guy. 2003. Optimization of aminolevulinic acid delivery by iontophoresis. J. Controlled Release. 88:65–70. [DOI] [PubMed] [Google Scholar]
- 14.Laksminarayanaiah, N. N. 1969. Transport Phenomena in Membranes. Academic Press, New York and London.
- 15.Luzardo-Alvarez, A., M. Rodríguez-Fernández, J. Blanco-Méndez, R. H. Guy, and M. B. Delgado-Charro. 1998. Iontophoretic permselectivity of mammalian skin: characterization of hairless mouse and porcine membrane models. Pharm. Res. 15:984–987. [DOI] [PubMed] [Google Scholar]
- 16.Marro, D., Y. N. Kalia, M. B. Delgado-Charro, and R. H. Guy. 2001. Optimizing drug delivery: identification and distribution of the charge-carrying species. Pharm. Res. 18:1709–1713. [DOI] [PubMed] [Google Scholar]
- 17.Kasting, G. B., and J. C. Keister. 1989. Application of electrodiffusion theory for a homogenous membrane to iontophoretic transport through skin. J. Controlled Release. 8:195–210. [Google Scholar]
- 18.Padmanabhan, R. V., J. B. Phipps, G. A. Lattin, and R. J. Sawchuk. 1990. In vitro and in vivo evaluation of transdermal iontophoretic delivery of hydromorphone. J. Controlled Release. 11:123–135. [Google Scholar]
- 19.Luzardo-Alvarez, A., M. B. Delgado-Charro, and J. Blanco-Méndez. 2001. Iontophoretic delivery of ropinirole hydrochloride: effect of current density and vehicle formulation. Pharm. Res. 18:1714–1720. [DOI] [PubMed] [Google Scholar]
- 20.Luzardo-Alvarez, A., M. B. Delgado-Charro, and J. Blanco-Méndez. 2003. In vivo iontophoretic administration of ropinirole hydrochloride. J. Pharm. Sci. 92:2441–2448. [DOI] [PubMed] [Google Scholar]
- 21.Roberts, M. S., P. M. Lai, and Y. G. Anissimov. 1998. Epidermal iontophoresis 1. Development of the ionic mobility-pore model. Pharm. Res. 15:1569–1578. [DOI] [PubMed] [Google Scholar]
- 22.Lai, M. P., and M. S. Roberts. 1998. Epidermal iontophoresis 2. Application of the ionic mobility-pore model to the transport of local anaesthetics. Pharm. Res. 15:1579–1588. [DOI] [PubMed] [Google Scholar]
- 23.Burnette, R. R., and B. Onpipattanakul. 1987. Characterization of the permselective properties of excised human skin during iontophoresis. J. Pharm. Sci. 76:765–773. [DOI] [PubMed] [Google Scholar]
- 24.Pikal, M. J., and S. Shah. 1990. Transport mechanisms in iontophoresis. II. Electroosmotic flow and transference number measurement for hairless mouse skin. Pharm. Res. 7:213–221. [DOI] [PubMed] [Google Scholar]
- 25.Glikfeld, P., R. S. Hinz, and R. H. Guy. 1989. Noninvasive sampling of biological fluids by iontophoresis. Pharm. Res. 6:988–990. [DOI] [PubMed] [Google Scholar]
- 26.Sieg, A., R. H. Guy, and M. B. Delgado-Charro. 2003. Reverse iontophoresis for noninvasive glucose monitoring: the internal standard concept. J. Pharm. Sci. 92:2295–2302. [DOI] [PubMed] [Google Scholar]
- 27.Turner, N. G., and R. H. Guy. 1997. Iontophoretic transport pathways: Dependence on penetrant physicochemical pathways. J. Pharm. Sci. 86:1385–1389. [DOI] [PubMed] [Google Scholar]
- 28.Delgado-Charro, M. B., and R. H. Guy. 2003. Transdermal reverse iontophoresis of valproate: A non-invasive tool for therapeutic drug monitoring. Pharm. Res. 20:1508–1513. [DOI] [PubMed] [Google Scholar]
- 29.Leboulanger, B., J. M. Aubry, G. Gondolfi, R. H. Guy, and M. B. Delgado-Charro. 2004. Non-invasive lithium monitoring by reverse iontophoresis in vivo. Clin. Chem. 50:2091–2100. [DOI] [PubMed] [Google Scholar]
- 30.Sieg, A., R. H. Guy, and M. B. Delgado-Charro. 2004. Electroosmosis in transdermal iontophoresis: implications for noninvasive and calibration-free glucose monitoring. Biophys. J. 87:3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falk, K. G. 1929. Transference numbers of electrolytes in aqueous solution. In International Critical Tables of Numerical Data, Physics, Chemistry and Technology, Vol. VI. E. W. Washburn, editor. McGraw-Hill, New York. 309–311.
- 32.Atkins, P. W. 1978. Molecules in motion: ion transport and molecular diffusion. In Physical Chemistry. P. W. Atkins, editor. Oxford University Press. Oxford, UK. 819–848.
- 33.Kasting, G. B., and L. A. Bowman. 1990. DC electrical properties of frozen, excised human skin. Pharm. Res. 7:134–143. [DOI] [PubMed] [Google Scholar]
- 34.Marro, D., R. H. Guy, and M. B. Delgado-Charro. 2001. Characterization of the iontophoretic permselectivity properties of human skin and pig skin. J. Controlled Release. 70:213–217. [DOI] [PubMed] [Google Scholar]
- 35.Green, P. G., R. S. Hinz, C. Cullander, G. Yamane, and R. H. Guy. 1991. Iontophoretic delivery of amino acids and amino acid derivatives across the skin in vitro. Pharm. Res. 8:1113–1120. [DOI] [PubMed] [Google Scholar]
- 36.Merino, V., A. López, Y. N. Kalia, and R. H. Guy. 1999. Electrorepulsion versus electroosmosis: effect of pH on the iontophoretic flux of 5-fluorouracil. Pharm. Res. 16:758–761. [DOI] [PubMed] [Google Scholar]
- 37.Kalia, Y. N., F. Pirot, R. O. Potts, and R. H. Guy. 1998. Ion mobility across human stratum corneum in vivo. J. Pharm. Sci. 87:1508–1511. [DOI] [PubMed] [Google Scholar]
- 38.Lentner, C. 1984. Geigy Scientific Tables. Ciba Geigy, Basel, Switzerland.
- 39.Sieg, A., R. H. Guy, and M. B. Delgado-Charro. 2004. Non-invasive glucose monitoring by reverse iontophoresis in vivo: application of the internal standard concept. Clin. Chem. 50:1383–1390. [DOI] [PubMed] [Google Scholar]
- 40.Marro, D., Y. N. Kalia, M. B. Delgado-Charro, and R. H. Guy. 2001. Contribution of electromigration and electroosmosis to iontophoretic drug delivery. Pharm. Res. 18:1701–1709. [DOI] [PubMed] [Google Scholar]