Abstract
Exposing bovine lipid extract surfactant (BLES), a clinical surfactant, to reactive oxygen species arising from hypochlorous acid or the Fenton reaction resulted in an increase in lipid (conjugated dienes, lipid aldehydes) and protein (carbonyls) oxidation products and a reduction in surface activity. Experiments where oxidized phospholipids (PL) were mixed with BLES demonstrated that this addition hampered BLES biophysical activity. However the effects were only moderately greater than with control PL. These results imply a critical role for protein oxidation. BLES oxidation by either method resulted in alterations in surfactant proteins SP-B and SP-C, as evidenced by altered Coomassie blue and silver staining. Western blot analyses showed depressed reactivity with specific antibodies. Oxidized SP-C showed decreased palmitoylation. Reconstitution experiments employing PL, SP-B, and SP-C isolated from control or oxidized BLES demonstrated that protein oxidation was more deleterious than lipid oxidation. Furthermore, addition of control SP-B can improve samples containing oxidized SP-C, but not vice versa. We conclude that surfactant oxidation arising from reactive oxygen species generated by air pollution or leukocytes interferes with surfactant function through oxidation of surfactant PL and proteins, but that protein oxidation, in particular SP-B modification, produces the major deleterious effects.
INTRODUCTION
Pulmonary surfactant is a complex mixture of lipids and proteins essential for normal lung function. Surfactant forms a film at the alveolar air-water interface that reduces the surface tension (γ), thereby reducing the work of breathing, protecting the alveoli against collapse at end-expiration, and reducing transudation of fluid from interstitial spaces (1–3). In addition to its surface-active properties, surfactant functions in the lung's host defense system and as an inflammatory reducing agent (4,5).
Surfactant chemical composition is conserved among most mammalian species, consisting of ∼80–90% phospholipids (PL), ∼2–10% neutral lipids, and ∼10% surfactant-associated proteins SP-A, SP-B, SP-C, and SP-D. The major surfactant PL is dipalmitoyl-phosphatidylcholine (DPPC; 35–40%), and, depending on the species, unsaturated PLs represent 50–60% of the weight of mammalian surfactants (6,7). The high proportion of DPPC is thought necessary for surfactant to be able to achieve γs near zero mN/m during the film compression occurring at expiration. In addition, it appears that surfactant needs unsaturated lipids to act as liquefiers for efficient adsorption to form a surface active film and for reinsertion of material during expansion of the film during the breathing cycle (3,7,8).
SP-A and SP-D belong to the collectin superfamily. SP-D locates primarily in the aqueous compartment of the epithelial lining layer, whereas SP-A is intimately associated with surfactant lipid aggregates such as lamellar bodies, vesicles, and tubular myelin (4,5). SP-A and SP-D have numerous roles in the lung's first-line host defense system. Both bind to a variety of pathogens, including bacteria, viruses, fungi, and yeasts, as well as lipopolysaccharides and allergens, and these proteins can modulate the production of reactive oxygen and nitrogen species (4,5).
SP-B, a sulphydryl-dependent homodimer of two 8.5-kDa monomers, is a membrane-associated protein, whereas SP-C (4.2 kDa) is a palmitoylated hydrophobic protein terminating in a C-terminal helix that functions as a transmembrane segment (9). SP-B and SP-C promote the adsorption of surfactant lipids to the air-liquid interface and stabilize the surfactant film during surface area reduction allowing it to attain γmins near zero mN/m. In addition, these hydrophobic proteins increase respreading of PL from collapse phase thereby allowing films to maintain γ near equilibrium during expansion (1–3,8–11).
Oxidative stress in the lung arises from environmental exposure to air pollutants and cigarette smoke and can also be a result of inflammation (12–14). Surfactant oxidative modifications and dysfunction likely play a central role in the pathogenesis of lung diseases, such as acute lung injury and acute respiratory distress syndrome (ARDS) (2,15). Numerous studies have confirmed that patients with ARDS show clear evidence of increased oxidative damage to lipids and proteins, as well as biophysical alterations of lung surfactant. For example, oxidative modifications of SP-A have been found in bronchoalveolar lavage of ARDS patients (16). Surfactant large aggregates isolated from ARDS patients exhibit high γmins relative to surfactant from controls (15).
Studies by various groups, including our own, have reported that exposure of natural, modified natural (clinical), or artificial surfactants to oxidative conditions resulted in decreased surface activity, including prolonged adsorption times and elevated γmin and γmax (17–21; see Rodríguez-Capote et al. for review (22)). The disruptive effects of reactive oxygen species (ROS) on surfactant biophysical activity have been usually attributed to alterations in surfactant lipids (18,21,23). Evidence indicating ROS damage to surfactant proteins has also been reported (17,24).
Despite considerable attention, the precise manner by which ROS affect surfactant PL and proteins, and which oxidative effects predominate, must still be established. To address these issues, we oxidized bovine lipid extract surfactant (BLES), a clinical surfactant containing all of the surfactant PL and the hydrophobic surfactant proteins SP-B and SP-C, with either hypochlorous acid or the Fenton reaction. A variety of analytical techniques were used to demonstrate biochemical modifications in both surfactant PL and hydrophobic proteins. Addition of oxidized phosphatidylcholine (PC) and phosphatidylglycerol (PG) molecular species to BLES hampered surface activity, but the overall effects were similar to those observed with control nonoxidized lipids. Further studies demonstrate that oxidation through either mechanism resulted in modification of SP-B and SP-C, resulting in alterations in the ability to interact with either Coomassie blue or silver staining. Hydrophobic protein interactions with specific antibodies were also affected. These observations prompted reconstitution studies using surfactant PL, SP-B, and SP-C isolated from control and oxidized BLES, which demonstrated that both PL and apoproteins are negatively influenced by oxidation. However, it became evident that the effects of ROS on the hydrophobic surfactant proteins have a greater influence on the surface activity than those on PL. Furthermore, the results indicate that oxidation of SP-B is more deleterious to surfactant function than oxidation of SP-C.
EXPERIMENTAL PROCEDURES
Reagents
All reagents were purchased from Sigma/Aldrich (Oakville, ON, Canada) and/or VWR Canlab (Mississauga, ON, Canada) unless otherwise noted. BLES was a kind gift of BLES Biochemicals (London, ON, Canada). Whatmann LK5 thin-layer plates with a concentration zone were employed for thin-layer chromatography (TLC).
DPPC was purchased from Sigma. 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC), 1-palmitoyl-2-hydroxy-sn-glycero-3-[phospho-rac-(1-glycerol)] sodium salt (LPG), 1-palmitoyl-2-oleoyl-sn-glycero-3 phospho-rac-(1-glycerol)-sodium salt (POPG), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PLPC), 1-palmitoyl-2-linoleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] sodium salt (PLPG), 1,2-diarachidonoyl-sn-glycero-3-phosphocholine (AAPC), and 1,2-diarachidonoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] sodium salt (AAPG) were obtained from Avanti Polar Lipids (Birmingham, AL).
Hypochlorous acid was purchased as sodium hypochlorite (NaOCl) from Sigma/Aldrich, specified with an active chlorine content of 10–13%. The hypochlorite concentration was determined spectrophotometrically immediately before use by diluting the NaOCl stock solution 1:10 in 1 M NaOH at 240 nm using a molar extinction coefficient for pH 12 of 43.6 mol−1 cm−1.
In vitro oxidation
For all oxidative treatments the reaction mixtures composed of 10 mg/ml surfactant lipids, plus Fenton reagents, hypochlorous acid, or controls in working buffer (in mM: 150 NaCl, 2 Tris-HCl, and 1.5 CaCl2 at pH 7.4), were incubated at 37°C in a shaking water bath for 24 h. Oxidation was halted by extraction with chloroform/methanol. For oxidation by the Fenton-like chemistry, BLES at 10 mg/ml was incubated with 0.65 mM FeCl2, 0.65 mM sodium ethylenediamine tetraacetic acid (EDTA), and 30 mM H2O2 in working buffer at a pH of 7.4 for 24 h (17,20). Fenton controls consisted of BLES in working buffer incubated with FeCl2/EDTA, H2O2, or alone. Treatment with HOCl/−OCl was carried out at a final concentration of 0.5 mM at pH 7.4 in working buffer (17,20).
Because the oxidizing conditions could theoretically affect measurement of lipid and/or protein concentrations, the PL and protein concentrations of triplicate surfactant samples were examined before and after oxidation, using the phosphorus assay (25) and the Lowry method (26,27), respectively. Total PL concentrations of hypochlorous-treated BLES (H∼BLES) and Fenton-treated BLES (F∼BLES) were 100.7 ± 1.5% and 102.1 ± 1.3%, respectively, of control BLES (C∼BLES). Resultant protein concentrations were 109.6 ± 0.4% for H∼BLES and 112.3 ± 1.3% for F∼BLES relative to C∼BLES. Neither PL nor protein concentrations were markedly influenced by the oxidative reactions; consequently, phosphorus estimations and Lowry protein levels were used to control the concentrations of whole BLES and reconstituted surfactants for surface activity measurements and biochemical analyses.
Phospholipid analyses
Lipid peroxidation
For primary lipoperoxidation products, conjugated dienes formed during oxidation were measured on diluted aliquots via spectrophotometric monitoring of the absorbance at 235 nm (A235) after exposure to HOCl/−OCl or Fenton reagents, as above. Aliquots of the control or oxidized BLES were diluted to 0.25 mg/ml of surfactant using working buffer. A235 was determined every 10 min for 5.5 h against a buffer blank.
For secondary lipoperoxidation products, the degree of lipid peroxidation during oxidation was determined by measuring the amounts of the secondary products malondialdehyde (MDA) and 4-hydroxynonenal (4HNE), using the lipid peroxidation assay kit BIOXYTECH (OXIS International) according to the manufacturer's instructions.
Thin layer chromatography
The PL samples in chloroform/methanol (1:1) were applied 20–200 μg in the concentration zone as 1-cm parallel streaks. Commercial phospholipids were used as standards. Plates developed with mobile phase consisting of chloroform/ethanol/water/triethylamine (30:35:7:35, v/v) (28). Individual PL subtypes were visualized either by molybdenum blue spray reagent (Sigma-Aldrich) or by the fluorescence dye primuline (0.05 mg/ml) (Sigma-Aldrich) dissolved in acetone:water (80:20, v/v) under low ultraviolet illumination. Fluorescent spots were scraped into glass tubes and the total PL content was determined by inorganic phosphate assay (25,28). The amount of phospholipids was calculated by multiplying the amount of Pi by 25.
Protein analyses
Protein isolation
Pulmonary SP-B and SP-C were isolated from BLES (BLES Biochemicals) by LH-60 chromatography as previously described (27,29). Protein concentrations were determined by a modification of the method of Lowry et al. (26,27). Correction factors of 2.0 for SP-B and 3.0 for SP-C relative to BSA were adopted on the basis of amino acid analysis as indicated previously (20,29,30). The purity of the proteins was routinely assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 18% gels. After isolation, surfactant proteins and PL were stored as chloroform/methanol: 0.1M HCl (1:1:0.05) solutions at -20°C until required.
Protein carbonyl concentration was determined by enzyme-linked immunosorbent assay (ELISA), using the Zentech PC Test Kit (Zenith Technology, Dunedin, New Zealand).
SP-B ELISA measurements were conducted using a mouse monoclonal antibody to human SP-B, kindly donated by Dr Y. Suzuki, Kyoto University, Japan, using the PL-soluble ELISA protocol developed by Kramer et al. (31). SP-B in the samples was calculated in comparison to purified bovine SP-B standard.
For SP-B western blot analysis, nonreduced samples (a total of 2 μg of protein per lane) were separated on SDS-14.5% PAGE and subsequently electroblotted onto hydrophobic polyvinylidene difluoride membranes (Immobilon-PSQ, Billerica, MA). SP-B was detected with a monoclonal antibody against SP-B (kindly donated by Dr Y. Suzuki), by employing enhanced chemiluminescence (SuperSignal West Femto from BioLynx, San Antonio, TX) and Kodak Biomax XAR film (Rochester, NY). This antiserum recognizes bovine, human, rabbit, and rat SP-B by western blotting and does not cross-react with SP-A, SP-C, rat serum proteins, or BSA.
For SP-C western blot analyses, one-dimensional SDS-PAGE was performed using Novex 10–20% tris-tricine gels (Invitrogen, Carlsbad, CA) at 70 V for 3 h. A total of 2 μg of protein (as determined by Lowry) was applied per lane. The gels were silver-stained using a commercially available kit (Bio-Rad, Rockville Center, NY). Separate (nonstained) gels were electrophoretically transferred onto Immobilon-PSQ membranes and immunoblotted using a monospecific rabbit antiserum for SP-C as the primary antibody (kind gift of Dr W. Steinhilber, Altana Pharma AG, Constance, Germany). Immunoreactive bands were visualized by chemiluminescence using Kodak Biomax XAR film.
For SP-C palmitoylation levels, a modification of the [14C] iodoacetamide assay developed by Qanbar et al. (32,33) was employed. Control and oxidized BLES and isolated SP-C from control and oxidized BLES (500 ng of total protein each) were mixed with triethylamine (TEA, 3 M) and 150 nCi of [14C] iodoacetamide (Dupont/NEN, Boston, MA) in chloroform-methanol (1:1), and the mixture (final volume, 170 μl) was incubated for 3 h at 37°C. After separation of the proteins on Novex precast 10–20% gradient Tricine gels, samples were transferred onto Immobilon-PSQ membranes. The relative amount of mature SP-C was determined by quantitation of [14C] SP-C bands with the software QuantiScan for Windows, BIOSOFT 99.
Captive bubble tensiometry
For the PL addition experiments, captive bubble tensiometer (CBT) assays were performed in triplicate, using 50 μg/ml BLES or BLES:PL samples dried under nitrogen and dispersed in working buffer. Adsorption, quasistatic, and dynamic experiments were conducted as described previously (20,29). Reconstituted samples were prepared by combining PL fractions from the column with isolated oxidized/nonoxidized 1% by weight SP-B and/or SP-C, also in organic solvent. This protein percent was adopted on the basis of preliminary studies performed with 0.5, 0.75, 1, and 1.5% of protein to find the optimal PL:protein ratio. For these studies, the lipid-protein mixtures were dried under a stream of nitrogen in Teflon tubes and then reconstituted in working buffer to a final concentration of 500 μg/ml. All samples were vortexed and shaken in an incubator with glass beads at 37°C for at least 1 h before studies on surface activity were initiated (29).
Statistical analysis
All experiments were performed in duplicate at least three separate times with individual freshly prepared samples. Statistical comparisons were conducted using SPSS (Chicago, IL) software. Comparisons among the three groups for the PL addition and the reconstitution studies were conducted by analysis of variance (ANOVA) followed by Bonferroni and Tukey post-hoc tests. Probability values <0.05 are considered significant.
RESULTS
Effects of free radical exposure on surfactant phospholipids
Initial studies examined the effects of free radical exposure on the lipid components of C∼BLES, F∼BLES, or H∼BLES. Conjugated diene formation was monitored during 5 h exposure of surfactant to the free radical generating systems (Fig. 1 A). C∼BLES (solid circles), BLES plus Fe+2:EDTA (open circles), or H2O2 (solid triangles) portrayed similar absorbance readings at 235 nm, which increased slightly over the first 60-min period and decreased slowly thereafter. When BLES was exposed to the complete Fenton reagents (solid squares) or to HOCl/−OCl (open triangles), there was an immediate increase in absorbance that reached a maximum around 60 min, after which it declined. Higher increases in A235 were manifested with the Fenton reaction compared to hypochlorous acid. The decrease in absorbance observed is consistent with the further modification of the conjugated dienes generated from susceptible unsaturated fatty acids and the production of secondary lipoperoxidation products such as MDA and 4HNE, which do not absorb ultraviolet.
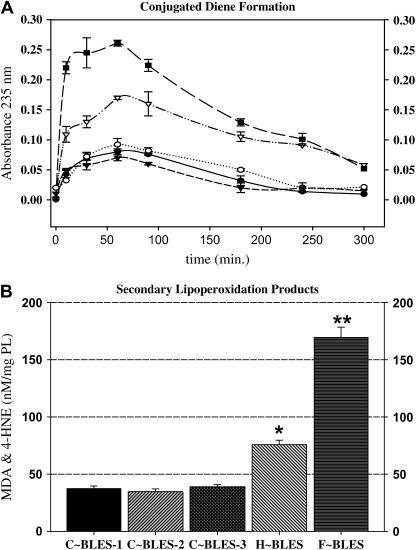
FIGURE 1.
Primary and secondary lipoperoxidation products. (A) The conjugated dienes formed during oxidation were monitored at 235 nm for 5 h (n = 3). Control samples are BLES in the absence of oxidants (C∼BLES-1, •); in the presence of H2O2 (C∼BLES-3, ○); and with Fe2+:EDTA (C∼BLES-2, ▾). Experimental samples included BLES in the presence of HOCl/−OCl (∇) and complete Fenton reagents (▪). (B) The presence of secondary lipoperoxidation products was tested as the content of MDA and 4HNE by using the lipoperoxidation assay kit (BIOXYTECH). Comparisons between two groups were made using paired Student t-test (*, p < 0.05; **, p < 0.001).
The presence of MDA and 4HNE was confirmed by analyzing the samples 24 h after reaction initiation. F∼BLES generated six times more MDA and HNE than its controls, whereas H∼BLES produced a fourfold increase over C∼BLES. These results (Fig. 1 B) are in overall agreement with the kinetics of the conjugated diene formation reported in this study and with previously published results reporting elevations in primary and secondary lipid peroxidation products after incubation of various surfactants with in vitro free radical generating systems (17,19,20,34).
TLC was used to examine PL classes, in particular to establish whether lysoPL, detected previously by mass spectrometry (20), was formed during F∼ or H∼ oxidation. Samples of BLES, oxidized BLES, and the PL fractions isolated by LH-60 chromatography were fractionated by TLC and the phosphorus content determined. Table 1 lists the amounts of each PL recovered, expressed as a percentage of the total. After 24 h of exposure to hypochlorous acid or the Fenton reagents, the PC content decreased by ∼20%. The decreases in PC were accompanied by increases in LPC.
TABLE 1.
Effect of BLES oxidation on phospholipid classes
| BLES | H-BLES | F-BLES | PL | H∼PL | F∼PL | |
|---|---|---|---|---|---|---|
| LPC | 1.37 ± 0.27 | 6.43 ± 1.11** | 5.03 ± 0.76** | 0.96 ± 0.12 | 11.08 ± 0.01** | 9.19 ± 0.063** |
| SM | 1.81 ± 0.35 | 1.89 ± 0.31 | 1.93 ± 0.40 | 1.59 ± 0.00 | 2.45 ± 0.03* | 1.75 ± 0.05 |
| PC | 79.76 ± 2.82 | 60.97 ± 2.17* | 58.88 ± 2.32** | 72.30 ± 1.98 | 49.50 ± 3.19** | 50.00 ± 2.23** |
| PE | 2.43 ± 0.55 | 3.30 ± 0.80 | 3.53 ± 0.62 | 0.49 ± 0.03 | 2.85 ± 0.09* | 6.27 ± 0.21** |
| LPG | 1.29 ± 0.29 | 3.75 ± 0.16** | 4.03 ± 0.77** | 1.32 ± 0.03 | 1.37 ± 0.04 | 1.41 ± 0.04 |
| PI | 1.04 ± 0.29 | 0.38 ± 0.16 | 0.35 ± 0.31 | 2.37 ± 0.19 | 3.55 ± 0.12 | 3.37 ± 0.03 |
| PG | 14.10 ± 1.10 | 9.02 ± 0.22** | 8.28 ± 0.26** | 11.39 ± 0.44 | 7.96 ± 0.28* | 6.27 ± 0.13* |
| LBPA | 1.68 ± 0.16 | 2.25 ± 0.06* | 2.23 ± 0.16* | 1.51 ± 0.49 | 0.67 ± 0.00 | 2.05 ± 0.10 |
| origin | 1.12 ± 0.25 | 7.36 ± 0.84* | 9.60 ± 0.65* | 1.56 ± 0.27 | 8.76 ± 0.24* | 8.39 ± 0.48* |
Control: H∼BLES and F∼BLES were extracted and the recovered PLs were separated on TLC before and after isolation by LH-60 chromatography. PL phosphorus was measured as indicated in Methods. Recoveries of applied lipid were: C∼BLES, 98.5 ± 1.5; H∼BLES, 87.8 ± 4.7; F∼BLES, 85.9 ± 6.4; C∼PL, 96.5 ± 8.2; H∼PL, 85.9 ± 6.4; F∼PL, 90.1 ± 3.4 (n = 3). Abbreviations: LPC, lysophosphatidylcholine; SM, sphingomyelin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; LPG, lysophosphatidylglycerol; LBPA, lyso-bis-phosphatidic acid.
p < 0.05; **p < 0.01.
Lung surfactant PG is richer in unsaturated fatty acids than surfactant PC. PG percentage contents decreased by 35–45%. This was usually associated with increases in percentage of LPG. It will be noted that the increases in LPC and LPG do not account in full for the reduction in PC and PG. Part of this deficit likely arose from streaking of oxidized PL on the TLC plates. This was clearly extenuated in the case of the isolated PLs. Although there is no proof, we believe that the increases in phosphatidylethanolamine (PE) and phosphatidylinositol (PI) observed with the isolated oxidized PLs reflects streaking of oxidized lipids. In addition, part of the decreased recoveries arose from the PL phosphorus remaining at the origin with oxidized samples. These effects likely obscured alterations in minor PL components such as PI and PE. No differences were detected between Fenton- and hypochlorous-reacted surfactants.
Effects of free radical exposure on surfactant proteins
Protein carbonyls are the most commonly measured products of protein oxidation in biological samples (52). Fig. 2 is a bar graph summarizing protein carbonyls measured by ELISA. Protein carbonyl concentration was increased in oxidized BLES compared to C∼BLES (p < 0.01), with protein carbonyls in F∼BLES six times higher than H∼BLES (p = 0.008). Isolated SP-B and SP-C from oxidized BLES also contained increased protein carbonyls when compared to their controls (p < 0.01). Differences between hypochlorous reaction and Fenton were found only for isolated SP-B, where the F∼SP-B revealed 2.6 times more protein carbonyls than H∼SP-B.
FIGURE 2.
Protein carbonyl derivatives. Protein carbonyl levels were determined by ELISA, using the Zentech PC Test Kit (Zenith Technology). BLES values are expressed per mg BLES protein, where BLES contains 2% protein w/w. SP-B and SP-C values refer to isolated protein. Comparisons between two groups were made using paired Student t-test. a, p < 0.05 (control versus treated); b, p < 0.05 (H∼ versus F∼).
Addition of unsaturated oxidized or nonoxidized PL to BLES
The observation that both surfactant lipids and proteins are modified during oxidation prompted investigations on the effects of adding oxidized PL to BLES on biophysical activity. For these experiments POPG, POPC, PLPC, PLPG, AAPC, and AAPG were exposed to either the Fenton or HOCl/−OCl in vitro free radical generating systems as used for BLES oxidation (20). After 24 h of incubation, control and oxidized PL were extracted and added to BLES in organic solvent (at 20% w/w). The surfactant mixtures were dried and resuspended in working buffer, and surface activity was studied in the CBT.
Initial film formation (Fig. 3) was slightly retarded by addition of control or oxidized PL to BLES, but only a few surfactant preparations (BLES:F∼AAPG, BLES:F∼AAPC, and BLES:H∼POPG) displayed adsorption times significantly greater than those of their respective controls. Addition of either control or oxidized PL disturbed γmin compared to BLES by around 8–10 mN/m (Fig. 4). However, BLES:C∼PL versus BLES:H∼/F∼PL did not show statistical differences. It should be recognized that the observed increases in γmin represent major decreases in surface activity during surface area reduction. It is possible, therefore, that smaller additions could show differences between control and oxidized lipids. The percent of surface area reduction necessary to achieve γmins ∼2 mN/m from equilibrium was also substantially affected by addition of either control or oxidized PL, and in this case oxidized PL had a greater effect. As a result, all mixtures BLES:H∼/F∼PL (except BLES:H∼/F∼PLPC) reached significance levels (p < 0.05) when compared with their matching BLES:C∼PL (Fig. 5). The γmaxs values, on the other hand, remained relatively stable (<30 mN/m) for all the samples studied (Fig. 4).
FIGURE 3.
Effects of addition of oxidized PL to BLES on the initial film formation. Horizontal bar graph representing the time required for the films to achieve equilibrium surface tension. Control or oxidized POPG, POPC, PLPC, PLPG, AAPC, or AAPG were added to 50 μg/ml of BLES at 20% w/w relative to surfactant PL. All measurements were performed in triplicate at 37°C. Comparisons among the samples were conducted by ANOVA followed by Bonferroni and Tukey post-hoc tests. (*, p < 0.05 of appropriate controls).
FIGURE 4.
Effects of addition of oxidized PL to BLES. Minimum (•) and maximum (○) γs reached by the samples during the 21st dynamic cycling. Samples are the same as in Fig. 3. All measurements were performed in triplicate at 37°C. Comparisons among the samples were conducted by ANOVA followed by Bonferroni and Tukey post-hoc tests (*, p < 0.05 of appropriate controls).
FIGURE 5.
Effect of addition of oxidized PL to BLES, surface area reduction. The bar graph depicts the percentage of surface area compression required for the films to attain a minimum surface tension near 0 from 20 mN/m during the 21st dynamic cycling. Samples are the same as in Fig. 3. Extrapolated compressions of >100% were estimated for those films that could not attain low minima during compression. Comparisons among the samples were conducted using Tukey's studentized range (highly significant difference) test. Letters A–D represent comparisons among samples within a cycle. Means with the same letter are not significantly different for p < 0.05.
SP-B analysis
The lack of substantial differences between the effects of oxidized and control PL on BLES surfactant biophysical activity prompted further examination of the effects of oxidation on the surfactant low-molecular-weight hydrophobic proteins. Fig. 6, A–C, shows a representative Coomassie blue-stained gel, a representative western blot analysis, and SP-B ELISA values for control and oxidized BLES as well as for the proteins isolated from C∼BLES, H∼BLES, and F∼BLES. Equal amounts of protein as estimated by PL phosphorus for each surfactant sample (C∼BLES, H∼BLES, and F∼BLES) and as detected by Lowry for the isolated proteins, were applied in each assay. The gel reveals that the oxidized SP-B band has decreased reactivity with Coomassie Blue stain, and this was more evident for Fenton-reacted BLES and for the post-Fenton isolated SP-B (Fig. 6 A). SP-B western blotting analysis (Fig. 6 B) demonstrated reduced chemiluminescence reactivity with the oxidized surfactant samples. The reduction of the signal was more evident for F∼SP-B. The ELISA values for SP-B (Fig. 6 C) confirmed the western blot findings for oxidized BLES and for isolated SP-B. The ELISA values not only quantitate the western blot results but test for potential differences due to alterations in molecular size arising from SP-B fragmentation.
FIGURE 6.
SP-B analysis. (A) Representative Coomassie blue stained gel, (B) representative western blot analysis, and (C) summary of SP-B ELISA values for control and oxidized BLES, as well as for the isolated proteins from BLES, H∼BLES, and F∼BLES. A total of 2 μg of protein as detected by Lowry were applied to each lane, as described in Experimental Procedures. Comparisons between two groups were made using paired Student t-test (*, p < 0.05; **, p < 0.01).
SP-C analysis
Fig. 7 shows Coomassie blue (A), silver-stained gels (B), and western blots (C) for control and oxidized BLES as well as for the SP-C isolated from BLES, H∼BLES, and F∼BLES. Equal amounts of protein as detected by Lowry were applied per lane. Because of the high concentration of PL in BLES or oxidized BLES, the amount of SP-C that could be detected by Coomassie blue was impaired and the protein was hardly visible. However the analysis of isolated SP-C shows that after oxidation, the ∼4-kDa band corresponding to this protein has decreased reactivity with Coomassie blue stain, and this was more evident for isolated F∼SP-C (Fig. 7 A). When the same gel was developed with silver stain (Fig. 7 B), a faint band corresponding to SP-C monomer was observed with C∼BLES (black arrow), but no corresponding bands were visible with either H∼BLES or F∼BLES. In addition, it was noted that SP-C from control BLES displayed lower silver stain reactivity than with Coomassie blue. Furthermore, it was observed that the isolated oxidized SP-Cs show higher degrees of polymerization with bands of molecular weight of ∼7 and 34 kDa appearing for H∼SP-C, and for F∼SP-C bands of ∼7, 55, 78, and 210 kDa. In addition, the band for SP-C isolated from F∼BLES corresponding to native SP-C (black arrow at ∼4 kDa) displayed more intense silver staining than the SP-C from C∼BLES. SP-C western blotting analysis (Fig. 7 C) demonstrated reduced chemiluminescence reactivity in the oxidized surfactant samples. The reduction in the signal was more evident for F∼BLES, H∼SP-C, and F∼SP-C.
FIGURE 7.
SP-C analysis. (A) Representative Coomassie blue gel and (B) representative silver-stained gel for control and oxidized BLES as well as for the isolated SP-C from C∼BLES, H∼BLES, and F∼BLES. A total of 2 μg of protein as detected by Lowry was applied per lane. Procedures are described in Experimental Procedures. The white arrows show the location of SP-B, whereas the black arrows show the location of SP-C. (C) Representative Western Blot confirming qualitative alterations of SP-C in the oxidized surfactants.
SP-C palmitoylation was estimated using reaction with [14C] iodoacetamide (32,33). Fig. 8 is a representative autoradiogram of 14C-labeled SP-Cs obtained from BLES, H∼BLES, and F∼BLES, as well as isolated C∼SP-C, H∼SP-C, and F∼SP-C. After the reacted samples were separated by Tricine-SDS-PAGE and transfered to nitrocellulose, a [14C] SP-C-labeled band corresponding to palmitoylated SP-C was detected. Control reactions with 500 ng of isolated bovine SP-C incubated without TEA are included to confirm specificity of the coupling reaction.
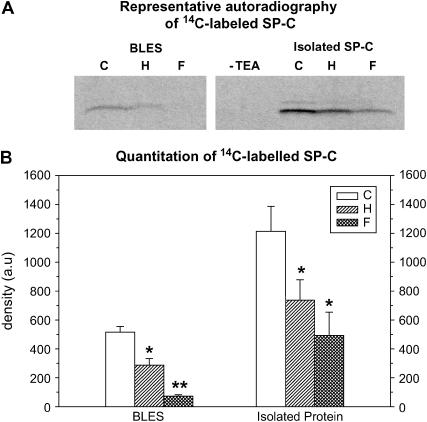
FIGURE 8.
SP-C palmitoylation. (A) Representative autoradiography of 14C-labeled SP-Cs obtained from BLES, H∼BLES, and F∼BLES, as well as isolated C∼SP-C, H∼SP-C, and F∼SP-C. After the reacted samples were separated by Tricine-SDS-PAGE and transferred to nitrocellulose, a [14C] SP-C-labeled band corresponding to palmitoylated SP-C was detected at 3.7 kDa. (B) Bar graph summarizing the relative amount of mature SP-C as determined by quantitation of [14C] SP-C bands with the software QuantiScan for Windows, BIOSOFT 99. Comparisons between two groups were made using paired Student t-test (*, p < 0.05; **, p < 0.01).
Fig. 8 B is a bar graph summarizing the quantitation of [14C] SP-C bands with the software QuantiScan for Windows, BIOSOFT 99. The autoradiography detected an ∼3.7-kDa band corresponding to mature dipalmitoylated SP-C and a smaller band, presumably corresponding to SP-C dimers, was also observed with the isolated C∼SP-C and H∼SP-C. Decreases in intensity of the bands due to reduced 14C incorporation were noticed for the oxidized samples indicating loss of SP-C palmitoylation (Fig. 8, A and B). Depalmitoylation of SP-C was greater with the Fenton reaction. These results are in agreement with those found by silver stain showing polymerization of SP-C since it has been reported that in vitro removal of palmitic acid from SP-C leads to destabilization of the protein and favors an increased rate of polymerization (11,35).
Effects of free radical exposure on surfactant function
The relative contributions of oxidized surfactant proteins SP-B and/or SP-C, and oxidized PL, to surfactant function impairment were studied with the CBT. Because of the large number of samples analyzed, not all of the surface activity results will be presented.
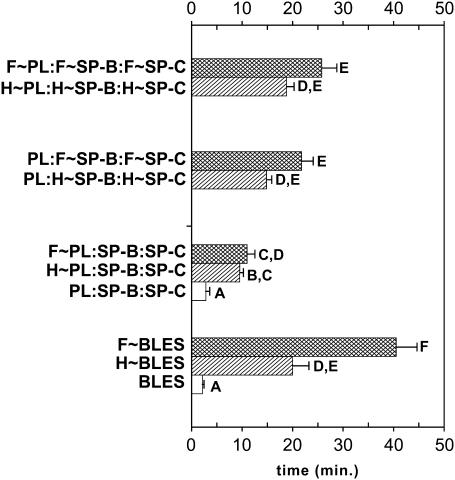
Total adsorption times to γeq for C∼BLES, H∼BLES, and F∼BLES (before chromatographic isolation of PL and proteins) and for the reconstituted samples studied are illustrated in Fig. 9. Oxidation by exposure to either of the two free radical systems resulted in retardation of initial film formation such that times to attain equilibrium increased from <5 min (C∼BLES) to ≈20 min (H∼BLES) and ≈40 min (F∼BLES). In agreement with previous studies (3,8,32), pure PL samples showed very slow adsorption. The films remained at 35–45 mN/m after 3 h and were not different from H∼PL or F∼PL (data not shown). The reconstituted surfactant containing PL:SP-B/SP-C adsorbs at a similar time to C∼BLES; likewise, the reconstituted samples containing all of the oxidized components or with both surfactant proteins oxidized paralleled the behavior of the parent surfactants H∼BLES and F∼BLES. Samples containing oxidized PL with nonoxidized proteins (H∼PL:SP-B/SP-C, F∼PL:SP-B/SP-C) also generated retarded adsorption times, but the effects were not as pronounced as with the oxidized proteins. When both oxidized proteins were present, the initial film formation was greatly impaired.
FIGURE 9.
Reconstitution studies. Effect of PL oxidation versus protein oxidation on surfactant adsorption. Horizontal bar graph illustrating the total adsorption times for C∼BLES, H∼BLES and F∼BLES before isolation and the following reconstituted samples in working buffer; control (PL:SP-B:SP-C), oxidized PL with nonoxidized proteins (H∼PL:SP-B:SP-C and F∼PL:SP-B:SP-C), both oxidized proteins with control PL (PL:H∼SP-B:H∼SP-C and PL:F∼SP-B:F∼SP-C) and all the constituents oxidized (H∼PL:H∼SP-B:H∼SP-C and F∼PL:F∼SP-B:F∼SP-C). Samples contain 500 μg PL/ml. Data are from at least three separate experimental samples. Comparisons among the samples were conducted using Tukey's studentized range test. Letters represent comparisons among samples within a cycle. Means with the same letter are not significantly different for p < 0.05.
Fig. 10 compares the effect of SP-B or SP-C oxidation on initial film formation. The presence of control SP-B or control SP-C alone or in combination was effective in promoting PL adsorption at the interface; adsorption times are indistinguishable from BLES (2.14 ± 0.31 min). When isolated SP-B from oxidized BLES, either F∼SP-B or H∼SP-B was present, addition of control SP-C did not improve this function. However, SP-B activity seems to prevail over oxidized SP-C, the samples PL:SP-B/H∼SP-C and PL:SP-B/F∼SP-C achieve γeq in ≈6 and 8 min, correspondingly, in opposition to PL:H∼SP-C and PL:F∼SP-C that adsorbed in ≈11 and 14 min, respectively, although these differences were not statistically significant. However, replacing H∼SP-B or F∼SP-B with control SP-B in the presence of the corresponding oxidized SP-C resulted in a significant reduction in adsorption time.
FIGURE 10.
Reconstitution studies. Effect of SP-B versus SP-C oxidation on surfactant adsorption. Horizontal bar graph illustrating the total adsorption times for controls (PL:SP-B, PL:SP-C and PL:SP-B:SP:C); samples containing oxidized SP-B (PL:H∼SP-B, PL:F∼SP-B, PL:H∼SP-B:SP-C, PL:F∼SP-B:SP-C); and samples containing oxidized SP-C, (PL:H∼SP-C, PL:F∼SP-C, PL:SP-B:H∼SP-C, and PL:SP-B:F∼SP-C). Samples are as in Fig. 9. Data are from at least three separate experimental samples. Comparisons among the samples were conducted by ANOVA followed by Bonferroni and Tukey post-hoc tests. Letters represent comparisons among samples. Means with the same letter are not significantly different for p < 0.05.
Dynamic cycling of adsorbed H∼/F∼BLES films resulted in considerably altered compression/expansion isotherms compared to C∼BLES. Initial isotherms for the adsorbed oxidized surfactants began from near 2–3 mN/m above γeq (23 mN/m), the films collapsed near γeq, and during film expansion, γmaxs rose above equilibrium such that the 21st compression cycle began at ∼40 mN/m for H-reacted and at >50 mN/m for F-reacted surfactant. As reported previously (3,29), lipid mixtures in the absence of proteins needed large compression ratios (>100% surface area reduction) and only achieved γmins of ∼18–20 mN/m with γmaxs ∼50 mN/m (data not shown).
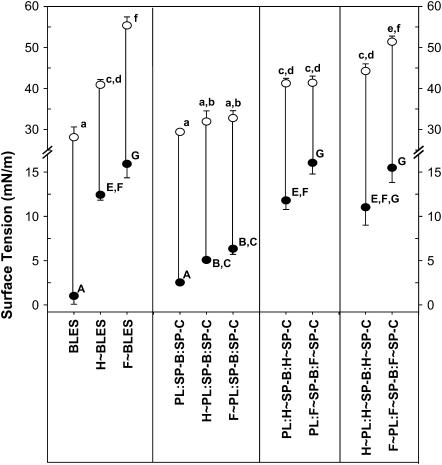
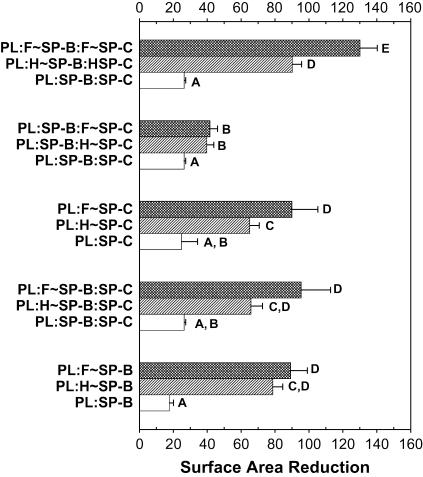
Figs. 11 and 12 represent the γmins (solid circles) and γmaxs (open circles) attained by the studied samples during the 21st dynamic cycle. PL:SP-B and PL:SP-B/SP-C achieved γmin of <5 mN/m, comparable to C∼BLES; conversely, the samples reconstituted with all of the oxidized components or both surfactant proteins oxidized (PL:H∼SP-B/H∼SP-C and PL:F∼SP-B/F∼SP-C) matched the elevated γmins of H∼BLES and F∼BLES. Mixtures containing oxidized PL but “good” proteins suffered modest elevations in γmin. When oxidized SP-B and SP-C were present, the films were not able to attain γmin <12 mN/m (Figs. 11 and 12). The most affected samples were those reconstituted with oxidized proteins isolated from F∼BLES. Samples containing PL:SP-B were almost as effective as BLES (Figs. 11 and 12). Replacing control SP-B with H∼SP-B or F∼SP-B elevated both γmin and γmax, and adding control SP-C had no effect. Samples containing PL:SP-C showed elevated γmins which increased with H∼SP-C or F∼SP-C. In each case, addition of control SP-B led to an improvement, which in the case of F∼SP-C was significant. In addition, replacing H∼ or F∼SP-B where both proteins were oxidized led to a significant improvement. Thus, similar to the initial film formation studies, SP-B activity seems to prevail over oxidized SP-C. However, the addition of “good” SP-C to surfactants containing oxidized SP-B could not rescue this function. These studies indicate that oxidizing either of the hydrophobic surfactant proteins leads to increased compressibility, but SP-B oxidation was more deleterious than SP-C oxidation.
FIGURE 11.
Reconstitution studies. Effect of PL oxidation versus protein oxidation on minimum and maximum γs. Minimum (•) and maximum (○) γs reached by the samples during the 21st dynamic cycling. Samples are as in Fig. 9. The data are the average of three separate experimental samples. Comparisons among the samples were conducted by ANOVA followed by Bonferroni and Tukey post-hoc tests. Letters a–f and A–G represent comparisons among samples in Figs 11 and 12. Means with the same letter are not significantly different for p < 0.05.
FIGURE 12.
Reconstitution studies. Effects of SP-B versus SP-C oxidation on minimum and maximum γs. Minimum (•) and maximum (○) γs reached by the samples during the 21st dynamic cycling. Samples are as in Fig. 10. The data are the average of three separate experimental samples. Comparisons among the samples were conducted by ANOVA followed by Bonferroni and Tukey post-hoc tests. Letters a–f and A–G represent comparisons among samples. Means with the same letter are not significantly different for p < 0.05.
Oxidation also affected BLES respreading. Whereas oxidized PL did not modify the γmaxs (>30 mN/m, Fig. 11) reached during film expansion, this parameter was significantly altered by the presence of oxidized proteins. The highest γmaxs were observed in samples reconstituted with oxidized SP-C and nonoxidized PL, reaching values >50 mN/m (Fig. 12). When SP-B isolated from BLES control was added to these samples, a reduction of ≈10 mN/m was recorded. However the addition of “good” SP-C to samples containing oxidized SP-B did not show improvement.
In our experience, the percent surface area reduction necessary to achieve γmins near zero mN/m is the most sensitive indication of good surfactant function. Oxidized BLES required a higher percent compression compared to BLES control (Fig. 13). When either PL or surfactant proteins were oxidized, the films required surface area reductions >50%. The highest surface area reductions were observed when all three components were oxidized. In all cases, Fenton reaction seemed to have a greater effect than hypochlorous reaction, although the differences were not always statistically significant. The effects of SP-B oxidation versus SP-C oxidation are summarized in Fig. 14. When SP-B or SP-C isolated from BLES control was added to control PL, low γs were achieved with a percent of compression <30%. However, when either oxidized SP-B or oxidized SP-C was added, larger percentage surface area reductions were required (60–100%). Although functional SP-B activity seems to prevail over oxidized SP-C, the addition of control SP-C to samples containing oxidized SP-B was not as effective in counteracting the effects of oxidized SP-B.
FIGURE 13.
Reconstitution studies. Effect of PL oxidation versus protein oxidation on surface area reduction. The bar graph shows the percentage of surface area reduction required for the films to attain a minimum surface tension near 0 from 20 mN/m during the 21st dynamic cycling. Extrapolated compressions of >100% were estimated for those films that could not attain low minima during compression. Samples are as in Fig. 9. Data are the average of three independent experiments. Comparisons among the samples were conducted using Tukey's studentized range (highly significant difference) test. Letters A–E represent comparisons among samples within Figs 13 and 14. Means with the same letter are not significantly different for p < 0.05.
FIGURE 14.
Reconstitution studies. Effect of SP-B and SP-C oxidation on surface area reduction. The bar graph shows the percentage of surface area reduction required for the films to attain a minimum surface tension near 0 from 20 mN/m during the 21st dynamic cycling. Extrapolated compressions of >100% were estimated for those films that could not attain low minima during compression. Samples are as in Fig. 10. The data are the average of three separate experimental samples. Comparisons among the samples were conducted using Tukey's studentized range (highly significant difference) test. Letters A–E represent comparisons among samples within a cycle. Means with the same letter are not significantly different for p < 0.05.
DISCUSSION
In vivo, pulmonary surfactant is directly exposed to oxidative air pollutants in the alveolar space. In the subphase between lung epithelial cells and the monolayer, surfactant is exposed to ROS produced by activated leukocytes and macrophages (22). ROS likely plays an important role in the pathogenesis of pulmonary diseases in adults (2,36) and preterm infants (37).
Activated neutrophils recruited into the alveolar space generate HOCl/−OCl (2,22,38). Surfactant molecules may be exposed also to ongoing Fenton chemistry. Pro-oxidant iron is present in normal human pulmonary epithelial lining fluid and was found increased in ARDS patients (39). Catalysis by Fe+2 in the generation of ROS through redox cycling has been well documented in biological systems, in tissue injuries, and in many pathological conditions (22,40).
In this study, we incubated BLES for 24 h with either hypochlorous acid or the Fenton reagents at concentrations considered in the pathophysiological range ((17,20,41), see Rodríguez-Capote et al. (22) for review). These two oxidizing systems produce different effects). Exposure to the Fenton reaction results in greater production of conjugated dienes, MDA and HNE, carbonyl derivatives, and protein modifications than exposure to HOCl/−OCl. These results are relevant, since there is growing evidence that many individual etiologies of ARDS are associated with a disruption of normal iron metabolism in the lung (39,42).
The unfavorable changes in surface activity after exposure of the surfactant samples to ROS suggested underlying mechanisms involving chemical reactions that can modify both PL and surfactant proteins and their interactions, thereby disturbing the balance and structural arrangements of the surfactant phospholipid-protein complexes and leading to poor surface activity. The TLC analysis showed that oxidized BLES and the PL fractions isolated from these oxidized surfactants contain significant decreases in total recoverable PC and PG, the most abundant PLs in surfactant, with consequent increases in LPC and, in the case of H∼ and F∼BLES, LPG. It is known that peroxidation of polyunsaturated PL acyl groups in vivo is accompanied by further chemical modifications leading to the generation of lysoPL via a phospholipase A2-like mechanism (19,43) with a complementary decrease in the fractional content of parental PL. LysoPL are amphipathic molecules possessing “detergent-like” properties that can alter cellular membranes and previous reports from our laboratory and others have shown that LPC inhibits surfactant activity (44–46). Likewise, the decrease in unsaturated species could also contribute to reduced surface activity (14,47). Unsaturated PGs appear to perform important functions in adsorption, spreading, and respreading of the film, likely by interactions of this anionic PL with SP-B ((29,48,49); see Possmayer (3) and Hawgood et al. (10) for review). Furthermore, decreases of PG and increases in lysoPL have been reported in inflammation-related airways disease ((50,51); see Lewis and Veldhuizen (2) and Gunther et al. (15) for review). The accumulation of lipoperoxidation subproducts such as aldehydes (MDA and HNE), free fatty acids, and lysoPL, as well as a decrease in unsaturated species may account in part for the reduced surface activity of oxidized BLES reported in this study.
PL addition to BLES affected surface activity, and for some surfactants the effects were greater when the PLs were oxidized. However, in general, no major differences in surface activity were found between BLES:C∼PL and BLES:H∼/F∼PL; only the percent of compression required to reach γs near zero show statistical differences between the groups. These results suggested that PL oxidation has minor detrimental effects on surface activity. This prompted us to examine in more detail the hypothesis that surfactant apoprotein oxidation is more important for the surfactant dysfunction.
Protein aggregation or fragmentation can occur during oxidation (52). However, although SP-B multimers have been reported (10), we were not able to detect higher- or lower-molecular-weight bands for SP-B in the gels by Coomassie blue or silver stain. Overall, similar decreases in reactivity were noted with BLES and with size-fractionated SP-B. Some gels (e.g., Fig. 8 B) gave evidence for altered migration. With such gels, control BLES showed a diffuse silver-stained band, likely due to the large amounts of lipid and the highly hydrophobic nature of SP-B. H∼BLES presents a similar, albeit diminished SP-B “shadow” with a smaller, more intensely stained band. A smaller shadowed band was observed with F∼BLES. In addition, because the amounts of protein applied were the same for each sample examined, ELISA and western blot results (Fig. 6, B and C) are consistent with the conclusion that oxidative modifications of SP-B led to decreased recognition by the anti-SP-B antibody. In each case, examination of isolated SP-B led to results similar to those obtained with whole surfactant. In all cases, SP-B structure/conformation was more affected by the Fenton reaction. SP-B contains amphipathic helices with the hydrophilic face containing a number of positively charged amino acids and a hydrophobic face rich in the aliphatic amino acids isoleucine, leucine, and valine (10). The amphipathic nature of SP-B allows the protein to interact with both the polar headgroups and the acyl side chains of the surfactant PL. Oxidized BLES shows increases in protein carbonyls, signifying decreases in basic amino acids such as His, Arg, and Lys (40,52), and consequently there are fewer positive charges available for the protein to interact with the PL. The loss of positive charges could contribute to the decrease in surface activity seen with SP-B isolated from oxidized BLES. In support of this suggestion, surfactant samples containing lipid and SP-B function less well in an alkaline environment than at pH values approaching the pKas of the basic amino acids (53,54).
SP-C contains two positively charged residues (Arg and Lys) in the N-terminal region that are important for the binding of PL vesicles to the monolayer, a process that precedes insertion of phospholipids into the monolayer (8,11). As mentioned above, these two amino acids are susceptible to forming protein carbonyls (52). Additional analysis of SP-C confirmed that this protein was further affected by the exposure to oxidation. The SDS-PAGE gel developed with silver stain shows appearance of higher-molecular-weight bands for the SP-C isolated from the oxidized samples. This may explain, in part, the decreased Coomasie blue staining intensity for the 3.7-kDa bands corresponding to the dipalmitoylated H∼SP-C (Fig. 7, A and B). Interestingly, although SP-C isolated from H∼BLES and F∼BLES shows reduced staining with Coomassie blue, SP-C from F∼BLES was highly reactive to silver staining. The mechanisms responsible for silver staining are complex (55), but it is evident from the gels that Fenton reaction increases rather than decreases reactivity. In agreement with these findings, oxidized SP-C portrayed lower levels of palmitoylation (Fig. 8). Palmitoylation of SP-C has been shown to be important for the stability and compressibility of the surfactant film as well as for the formation of the surface associated lipid reservoir (3,9,11). Under oxidative stress conditions, destabilizing amino acid modifications and cleavage of the SP-C two thioester-linked palmitoyl groups may be mediated by ROS, such as hydrogen peroxide, superoxide anions, peroxynitrite, and hypochlorite released from inflammatory cells. The loss of the palmitoyl groups and/or loss of the two positive charges provoked by oxidation may destabilize SP-C, which can influence amyloid formation by facilitating α-helix to β-sheet conversion, resulting in fibril formation (11,35). Whether the decreased SP-C activity documented here arises from depalmitoylation or from amino acid modifications resulting in oligomerization and decreased anti-SP-C antibody recognition is not clear from the studies described here, and awaits further investigation.
The mechanisms by which pulmonary surfactant functions are not fully understood. Nevertheless it is clear that the surfactant proteins SP-B and SP-C are essential for surfactant function. In vitro experiments conducted with spread surfactant monolayers suggested that these surfactant apoproteins alter the PL packing, lowering the compressibility of the monolayer. A key role of SP-B and SP-C is to alter the collapse mechanism from fracture and vesicle formation to a reversible folding that allows the films to achieve low γ during surface area reduction while favoring respreadability during surface area expansion (8,9,56). It is now believed that in vivo a multilayer of lipid-protein structures underlying the surface monolayer expands and contracts as material leaves and reenters the monolayer during the respiratory cycles, and that the surfactant proteins contribute to this behavior (56–59). When either oxidized SP-B or SP-C was present, surface activity was greatly affected. These results are in good overall agreement with previous studies demonstrating that exposing surfactant or surfactant proteins to reactive oxygen or nitrogen species results in reduced surfactant activity (18,24). Interesting results were obtained when SP-B or SP-C isolated from BLES control was added to samples reconstituted with control PL and the other protein oxidized. Addition of control SP-B to samples containing oxidized SP-C resulted in a significant improvement in adsorption, γ reduction properties, and respreading. In other words, the activity of “good” SP-B seems to prevail over the deleterious effects of oxidized SP-C. However, the presence of “good” SP-C in samples containing oxidized SP-B could not rescue the surface activity. In this regard, it should be mentioned that addition of SP-A to H∼BLES and F∼BLES results in a significant recovery of surface activity (20). Based on parallel studies, this SP-A-induced improvement is likely dependent on the presence of SP-B (29).
These investigations demonstrated that ROS arising from either hypochlorous acid or the Fenton reaction affected pulmonary surfactant structure and function. Interestingly, under the conditions employed, Fenton had a significantly greater effect on PL and surfactant protein chemistry than hypochlorous acid. Differences in the effects on surfactant function were less obvious. However F∼reacted SP-B and/or SP-C were less effective than H∼reacted counterparts in film compressibility. This is the most sensitive and likely the most critical parameter studied. Thus, we conclude that the Fenton reaction affects both surfactant structure and function to a greater extent than hypochlorous acid.
The fundamental importance of SP-B in pulmonary function in vivo is emphasized by the observation that infants unable to produce SP-B due to mutations of the SP-B gene develop lethal neonatal respiratory disease (60). Antibodies to SP-B disrupt surfactant function in vivo (3,61). Targeted disruption of the SP-B gene in mice results in respiratory failure and death immediately after birth in the homozygous SP-B-deficient mice because of their inability to inflate their lungs and establish respiration (61,62). Genetic curtailing of SP-B production in adult mice induces lethal respiratory distress associated with loss of function of the pulmonary surfactant (62). Whereas SP-B deficiency results in an understandable phenotype, the lung diseases associated with heterozygous SP-C deficiency as well as the functions of SP-C in vivo are not fully understood. Although no obvious abnormalities were identified in SP-C knockout mice at birth, they showed signs of surfactant instability at low lung volumes and in adulthood they developed pneumonia and emphysema (61,63). SP-C alterations have been associated with chronic lung diseases in humans (60,63). It is possible that modifications of SP-C, whether genetic or secondary to oxidative damage, are masked because the presence of SP-B guarantees acceptable surfactant activity for the performance of respiratory cycles. Consequently, SP-C alterations contribute to chronic lung diseases only in an accumulative manner, most likely through cell toxicity.
In summary, the results described in this article reveal that, in addition to surfactant lipids, both SP-B and SP-C undergo changes during oxidation that significantly decrease their biophysical properties. The surface activity impairment found in the reconstituted mixtures suggests that protein oxidation is the major cause of the impaired activity of oxidized surfactants, whereas PL oxidation has a lesser effect. Although oxidation to either SP-B or SP-C can hamper surfactant function, nonoxidized SP-B can improve samples containing oxidized SP-C. Damage to surfactant hydrophobic proteins may play an important role in the surfactant dysfunction that arises during lung oxidative stress-related disorders such as ARDS, cystic fibrosis, and asthma.
Acknowledgments
The authors express their gratitude to BLES Biochemicals for providing the BLES surfactant used in these studies. In addition, they thank Dr. Y. Suzuki, Kyoto University, Japan, for providing the monoclonal antibody against the human SP-B, and Dr. W. Steinhilber, Altana Pharma AG, Constance, Germany, for the rabbit anti-SP-C antibody. We acknowledge the technical assistance and advice of Ms. Anne Brickenden.
This work was supported by a Group Grant (MOP15264) and an Operating Grant (MOP64406) from the Canadian Institutes of Health Research.
Dr. Dahis Manzanares' present address is Div. of Pulmonary and Critical Care Medicine, University of Miami, School of Medicine, Miami, FL.
References
- 1.Bachofen, H., and S. Schurch. 2001. Alveolar surface forces and lung architecture. Comp. Biochem. Physiol. A. 129:183–193. [DOI] [PubMed] [Google Scholar]
- 2.Lewis, J. F., and R. Veldhuizen. 2003. The role of exogenous surfactant in the treatment of acute lung injury. Annu. Rev. Physiol. 65:613–642. [DOI] [PubMed] [Google Scholar]
- 3.Possmayer, F. 2004. Physicochemical aspects of pulmonary surfactant. In Fetal and Neonatal Physiology, 3rd ed. R. A. Polin, W. W. Fox, and S. H. Abman, editors. W. B. Saunders, Philadelphia. 1014–1034.
- 4.Crouch, E., and J. R. Wright. 2001. Surfactant proteins A and D and pulmonary host defense. Annu. Rev. Physiol. 63:521–554. [DOI] [PubMed] [Google Scholar]
- 5.McCormack, F. X., and J. A. Whitsett. 2002. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Invest. 109:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postle, A. D., E. L. Heeley, and D. C. Wilton. 2001. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129:65–73. [DOI] [PubMed] [Google Scholar]
- 7.Veldhuizen, R., K. Nag, S. Orgeig, and F. Possmayer. 1998. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta. 1408:90–108. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Gil, J., and K. M. W. Keough. 1998. Interfacial properties of surfactant proteins. Biochim. Biophys. Acta. 1408:203–217. [DOI] [PubMed] [Google Scholar]
- 9.Possmayer, F., K. Nag, K. Rodriguez, R. Qanbar, and S. Schurch. 2001. Surface activity in vitro: role of surfactant proteins. Comp. Biochem. Physiol. A. 129:209–220. [DOI] [PubMed] [Google Scholar]
- 10.Hawgood, S., M. Derrick, and F. Poulain. 1998. Structure and properties of surfactant protein B. Biochim. Biophys. Acta. 1408:150–160. [DOI] [PubMed] [Google Scholar]
- 11.Johansson, J. 1998. Structure and properties of surfactant protein C. Biochim. Biophys. Acta. 1408:161–172. [DOI] [PubMed] [Google Scholar]
- 12.Bowler, R. P., and J. D. Crapo. 2002. Oxidative stress in allergic respiratory diseases. J. Allergy Clin. Immunol. 110:349–356. [DOI] [PubMed] [Google Scholar]
- 13.Heeley, E. L., J. M. Hohlfeld, N. Krug, and A. D. Postle. 2000. Phospholipid molecular species of bronchoalveolar lavage fluid after local allergen challenge in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L305–L311. [DOI] [PubMed] [Google Scholar]
- 14.Putman, E., L. M. van Golde, and H. P. Haagsman. 1997. Toxic oxidant species and their impact on the pulmonary surfactant system. Lung. 175:75–103. [DOI] [PubMed] [Google Scholar]
- 15.Gunther, A., C. Ruppert, R. Schmidt, P. Markart, F. Grimminger, D. Walmrath, and W. Seeger. 2001. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir. Res. 2:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu, S., L. B. Ware, T. Geiser, M. A. Matthay, and S. Matalon. 2001. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am. J. Respir. Crit. Care Med. 163:166–172. [DOI] [PubMed] [Google Scholar]
- 17.Andersson, S., A. Kheiter, and T. A. Merritt. 1999. Oxidative inactivation of surfactants. Lung. 177:179–189. [DOI] [PubMed] [Google Scholar]
- 18.Gilliard, N., G. P. Heldt, J. Loredo, H. Gasser, H. Redl, T. A. Merritt, and R. G. Spragg. 1994. Exposure of the hydrophobic components of porcine lung surfactant to oxidant stress alters surface tension properties. J. Clin. Invest. 93:2608–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark, L., and E. P. Ingenito. 1999. Surfactant function and composition after free radical exposure generated by transition metals. Am. J. Physiol. 276:L491–L500. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Capote, K., F. X. McCormack, and F. Possmayer. 2003. Pulmonary surfactant protein-A (SP-A) restores the surface properties of surfactant after oxidation by a mechanism that requires the Cys6 interchain disulfide bond and the phospholipid binding domain. J. Biol. Chem. 278:20461–20474. [DOI] [PubMed] [Google Scholar]
- 21.Seeger, W., H. Lepper, H. R. Wolf, and H. Neuhof. 1985. Alteration of alveolar surfactant function after exposure to oxidative stress and to oxygenated and native arachidonic acid in vitro. Biochim. Biophys. Acta. 835:58–67. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Capote, K., J. Faulkner, K. Nag, and F. Possmayer. 2005. Alteration of alveolar surfactant function by reactive oxygen species. In Lung Surfactant Function and Disorder. K. Nag, editor. Marcel Dekker, New York. 425–448.
- 23.Lee, M. M., F. H. Y. Green, S. Schurch, S. Cheng, S. G. Bjarnason, S. Leonard, W. Wallace, F. Possmayer, and V. Vallyathan. 2004. Comparison of inhibitory effects of oxygen radicals and calf serum protein on surfactant activity. Mol. Cell. Biochem. 259:15–22. [DOI] [PubMed] [Google Scholar]
- 24.Haddad, I. Y., H. Ischiropoulos, B. A. Holm, J. S. Beckman, J. R. Baker, and S. Matalon. 1993. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am. J. Physiol. 265:L555–L564. [DOI] [PubMed] [Google Scholar]
- 25.Rouser, G., S. Fleischer, and A. Yamamoto. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids. 5:494–496. [DOI] [PubMed] [Google Scholar]
- 26.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- 27.Qanbar, R., S. Cheng, F. Possmayer, and S. Schurch. 1996. Role of the palmitoylation of surfactant-associated protein C in surfactant film formation and stability. Am. J. Physiol. 271:L572–L580. [DOI] [PubMed] [Google Scholar]
- 28.Yu, S., P. G. Harding, N. Smith, and F. Possmayer. 1983. Bovine pulmonary surfactant: chemical composition and physical properties. Lipids. 18:522–529. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez Capote, K., K. Nag, S. Schurch, and F. Possmayer. 2001. Surfactant protein interactions with neutral and acidic phospholipid films. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L231–L242. [DOI] [PubMed] [Google Scholar]
- 30.Yu, S. H., W. Chung, and F. Possmayer. 1989. Structural relationship between the two small hydrophobic apoproteins in bovine pulmonary surfactant. Biochim. Biophys. Acta. 1005:93–96. [DOI] [PubMed] [Google Scholar]
- 31.Kramer, H. J., R. Schmidt, A. Gunther, G. Becker, Y. Suzuki, and W. Seeger. 1995. ELISA technique for quantification of surfactant protein B (SP-B) in bronchoalveolar lavage fluid. Am. J. Respir. Crit. Care Med. 152:1540–1544. [DOI] [PubMed] [Google Scholar]
- 32.Atochina, E. N., M. F. Beers, S. T. Scanlon, A. M. Preston, and J. M. Beck. 2000. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L599–L609. [DOI] [PubMed] [Google Scholar]
- 33.Qanbar, R., and F. Possmayer. 1994. A quantitative method for detecting surfactant-associated protein C in pulmonary surfactant. Anal. Biochem. 216:262–270. [DOI] [PubMed] [Google Scholar]
- 34.Marzan, Y., R. Mora, A. Butler, M. Butler, and E. P. Ingenito. 2002. Effects of simultaneous exposure of surfactant to serum proteins and free radicals. Exp. Lung Res. 28:99–121. [DOI] [PubMed] [Google Scholar]
- 35.Johansson, J., T. E. Weaver, and L. O. Tjernberg. 2004. Proteolytic generation and aggregation of peptides from transmembrane regions: lung surfactant protein C and amyloid beta-peptide. Cell. Mol. Life Sci. 61:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamb, N. J., J. M. Gutteridge, C. Baker, T. W. Evans, and G. J. Quinlan. 1999. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit. Care Med. 27:1738–1744. [DOI] [PubMed] [Google Scholar]
- 37.Saugstad, O. D. 2001. Update on oxygen radical disease in neonatology. Curr. Opin. Obstet. Gynecol. 13:147–153. [DOI] [PubMed] [Google Scholar]
- 38.Downey, G. P., Q. Dong, J. Kruger, S. Dedhar, and V. Cherapanov. 1999. Regulation of neutrophil activation in acute lung injury. Chest. 116:46S–54S. [PubMed] [Google Scholar]
- 39.Gutteridge, J. M., G. J. Quinlan, and T. W. Evans. 2001. The iron paradox of heart and lungs and its implications for acute lung injury. Free Radic. Res. 34:439–443. [DOI] [PubMed] [Google Scholar]
- 40.Stadtman, E. R., and R. L. Levine. 2003. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 25:207–218. [DOI] [PubMed] [Google Scholar]
- 41.Amirkhanian, J. D., and T. A. Merritt. 1998. Inhibitory effects of oxyradicals on surfactant function: utilizing in vitro Fenton reaction. Lung. 176:63–72. [DOI] [PubMed] [Google Scholar]
- 42.Quinlan, G. J., T. W. Evans, and J. M. Gutteridge. 2002. Iron and the redox status of the lungs. Free Radic. Biol. Med. 33:1306–1313. [DOI] [PubMed] [Google Scholar]
- 43.Arnhold, J., A. N. Osipov, H. Spalteholz, O. M. Panasenko, and J. Schiller. 2002. Formation of lysophospholipids from unsaturated phosphatidylcholines under the influence of hypochlorous acid. Biochim. Biophys. Acta. 1572:91–100. [DOI] [PubMed] [Google Scholar]
- 44.Cockshutt, A. M., and F. Possmayer. 1991. Lysophosphatidylcholine sensitizes lipid extracts of pulmonary surfactant to inhibition by serum proteins. Biochim. Biophys. Acta. 1086:63–71. [DOI] [PubMed] [Google Scholar]
- 45.Enhorning, G., B. Shumel, L. Keicher, J. Sokolowski, and B. A. Holm. 1992. Phospholipases introduced into the hypophase affect the surfactant film outlining a bubble. J. Appl. Physiol. 73:941–945. [DOI] [PubMed] [Google Scholar]
- 46.Holm, B. A., Z. Wang, and R. H. Notter. 1999. Multiple mechanisms of lung surfactant inhibition. Pediatr. Res. 46:85–93. [DOI] [PubMed] [Google Scholar]
- 47.Zhu, S., M. Manuel, S. Tanaka, N. Choe, E. Kagan, and S. Matalon. 1998. Contribution of reactive oxygen and nitrogen species to particulate-induced lung injury. Environ. Health Perspect. 106(Suppl 5):1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serrano, A. G., A. Cruz, K. Rodriguez-Capote, F. Possmayer, and J. Perez-Gil. 2005. Intrinsic structural and functional determinants within the amino acid sequence of mature pulmonary surfactant protein SP-B. Biochemistry. 44:417–430. [DOI] [PubMed] [Google Scholar]
- 49.Yu, S.-H., and F. Possmayer. 1992. Effect of pulmonary surfactant protein B (SP-B) and calcium on phospholipid adsorption and squeeze-out of phosphatidylglycerol from binary phospholipid monolayers containing dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta. 1126:26–34. [DOI] [PubMed] [Google Scholar]
- 50.Hite, R. D., M. C. Seeds, D. L. Bowton, B. L. Grier, A. M. Safta, R. Balkrishnan, B. M. Waite, and D. A. Bass. 2005. Surfactant phospholipid changes after antigen challenge: a role for phosphatidylyglycerol in dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L610–L617. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt, R., U. Meier, M. Yabut-Perez, D. Walmrath, F. Grimminger, W. Seeger, and A. Gunther. 2001. Alteration of fatty acid profiles in different pulmonary surfactant phospholipids in acute respiratory distress syndrome and severe pneumonia. Am. J. Respir. Crit. Care Med. 163:95–100. [DOI] [PubMed] [Google Scholar]
- 52.Shacter, E. 2000. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 32:307–326. [DOI] [PubMed] [Google Scholar]
- 53.Haddad, I. Y., B. A. Holm, L. Hlavaty, and S. Matalon. 1994. Dependence of surfactant function on extracellular pH: mechanisms and modifications. J. Appl. Physiol. 76:657–662. [DOI] [PubMed] [Google Scholar]
- 54.Metcalfe, I. L., G. Enhorning, and F. Possmayer. 1980. Pulmonary surfactant-associated proteins: their role in the expression of surface activity. J. Appl. Physiol. 49:34–41. [DOI] [PubMed] [Google Scholar]
- 55.Gersten, D. M., L. V. Rodriguez, D. G. George, D. A. Johnston, and E. J. Zapolski. 1991. On the relationship of amino acid composition to silver staining of proteins in electrophoresis gels: II. Peptide sequence analysis. Electrophoresis. 12:409–414. [DOI] [PubMed] [Google Scholar]
- 56.Takamoto, D. Y., M. M. Lipp, A. von Nahmen, K. Y. Lee, A. J. Waring, and J. A. Zasadzinski. 2001. Interaction of lung surfactant proteins with anionic phospholipids. Biophys. J. 81:153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schurch, S., H. Bachofen, and F. Possmayer. 2001. Surface activity in situ, in vivo, and in the captive bubble surfactometer. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129:195–207. [DOI] [PubMed] [Google Scholar]
- 58.Schurch, S., F. H. Y. Green, and H. Bachofen. 1998. Formation and structure of surface films: captive bubble surfactometry. Biochim. Biophys. Acta. 1408:180–202. [DOI] [PubMed] [Google Scholar]
- 59.Schurch, S., R. Qanbar, H. Bachofen, and F. Possmayer. 1995. The surface-associated surfactant reservoir in the alveolar lining. Biol. Neonate. 67:61–76. [DOI] [PubMed] [Google Scholar]
- 60.Nogee, L. M. 2004. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu. Rev. Physiol. 66:601–623. [DOI] [PubMed] [Google Scholar]
- 61.Whitsett, J. A., and T. E. Weaver. 2002. Hydrophobic surfactant proteins in lung function and disease. N. Engl. J. Med. 347:2141–2148. [DOI] [PubMed] [Google Scholar]
- 62.Melton, K. R., L. L. Nesslein, M. Ikegami, J. W. Tichelaar, J. C. Clark, J. A. Whitsett, and T. E. Weaver. 2003. SP-B deficiency causes respiratory failure in adult mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L543–L549. [DOI] [PubMed] [Google Scholar]
- 63.Beers, M. F., and S. Mulugeta. 2005. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu. Rev. Physiol. 67:663–696. [DOI] [PubMed] [Google Scholar]