Abstract
Cortical neurons and astrocytes respond strongly to changes in matrix rigidity when cultured on flexible substrates. In this study, existing polyacrylamide gel polymerization methods were modified into a novel method for making substrates capable of engaging specific cell-adhesion receptors. Embryonic cortical dissociations were cultured on polyacrylamide or fibrin gel scaffolds of varying compliance. On soft gels, astrocytes do not spread and have disorganized F-actin compared to the cytoskeletons of astrocytes on hard surfaces. Neurons, however, extend long neurites and polymerize actin filaments on both soft and hard gels. Compared to tissue culture plastic or stiff gel substrates coated with laminin, on which astrocytes overgrow neurons in mixed cultures, laminin-coated soft gels encourage attachment and growth of neurons while suppressing astrocyte growth. The number of astrocytes on soft gels is lower than on hard even in the absence of mitotic inhibitors normally used to temper the astrocyte population. Dissociated embryonic rat cortices grown on flexible fibrin gels, a biomaterial with potential use as an implant material, display a similar mechano-dependent difference in cell population. The stiffness of materials required for optimal neuronal growth, characterized by an elastic modulus of several hundred Pa, is in the range measured for intact rat brain. Together, these data emphasize the potential importance of material substrate stiffness as a design feature in the next generation of biomaterials intended to promote neuronal regeneration across a lesion in the central nervous system while simultaneously minimizing the ingrowth of astrocytes into the lesion area.
INTRODUCTION
Injury to the central nervous system (CNS) is characterized by a lesion cavity surrounded by a glial scar composed primarily of hypertrophic astrocytes, potentially as a means to seal off the damaged area from still healthy tissue (1). Neuronal regeneration through the lesion cavity is only marginally feasible, perhaps due to many obstacles including a severe injury environment filled with deleterious factors and the mechanical barrier caused by the glial scar (2). Less well known, though, is the mechanical influence of the glial scar on the regenerative response across the lesion site.
Cells are subject not only to chemical signals but also to mechanical signals (3–6). Numerous systems developed to define how cultured cells respond when grown on materials of physiologically realistic elastic moduli show that mechanical inputs matter to cells and that their response varies with cell type (7–9). Plating cells on hydrogels with controllable stiffness has identified how mechanical parameters affect cell behavior (3). Endothelial cells, fibroblasts, myotubes, smooth muscle cells, as well as neurons all show a morphological dependence on matrix stiffness (3,7,8,10–12). Neurons of the dorsal root ganglion and motor neurons of the spinal cord exhibit a preference for growth on soft materials by increased neurite length and increased branching, respectively (8,10). Furthermore, implanting soft hydrogels into the CNS injury cavity has beneficial effects including modest neuronal ingrowth as well as reduction of the glial scar (13). The benefit provided by hydrogels may partly be attributable to their mechanical properties (14–16). Whether extracellular compliance affects specific aspects of neuronal growth in culture with other CNS cell types such as astrocytes has not yet been determined.
The work presented here attempts to link matrix compliance to growth behavior, adhesion, and morphology of primary cortical neurons and astrocytes. In normal culture conditions on hard polystyrene, dissociation of brain or spinal cord yields a mixed culture of both glia and neurons. Most efforts to maximize neuronal growth have focused on identifying growth-promoting or inhibitory chemical signals, but the mechanical properties of plastic substrates with elastic moduli in the GPa range also contrasts with the mechanical properties of the brain, which has elastic moduli in the few hundred Pa range (17,18). Altering the matrix stiffness to values above and below physiological levels shifts the resulting cell populations on the gel surface. Our data indicate that neurons show a bias for growth on soft materials, whereas astrocytes spread and adhere better to stiff materials. Appreciating the effects of matrix mechanics on CNS cell growth could help characterize factors regulating neuronal growth and the development of biomaterial scaffolds used to bridge the lesion gap that occurs after traumatic CNS injury.
EXPERIMENTAL METHODS
Preparation of substrates
Acrylamide and bis-acrylamide (Fisher Biotech, Loughborough, Leicestershire, UK) solutions are prepared to contain a constant polymer mass of 7.5% and bis-acrylamide concentrations of 0.01%, 0.03% or 0.3% to alter stiffness. Acrylamide, bis-acrylamide, ammonium persulfate, and N,N,N′,N′-tetramethylethylenediamine (TEMED) under a nonaqueous layer of toluene containing 0.5% acrylic acid N-hydroxy succinimide ester (Sigma, St. Louis, MS) was polymerized between two coverslips, chemically modified as described (12). The N-succinimidyl acrylate incorporated at the surface of the gel was reacted with 0.2 mg/ml laminin (Collaborative Biomedical, Bedford, MA) to produce a uniform coating of adhesive ligands. After washing with HEPES buffer to remove traces of unpolymerized solvent, wells containing the polyacrylamide (PA) gels were filled with culture medium and allowed to equilibrate overnight at 37°C.
Although the PA gels represent a well-controlled experimental system to vary both matrix compliance and surface coatings, the PA polymerization process prevents its use as an implantable biomaterial. An alternative choice with potential as an implant material is fibrin, which was polymerized using standard methods (19). Salmon fibrinogen (Searun Holdings, Freeport, ME) was rehydrated in dH2O and diluted to 2 or 18 mg/mL in 50 mM Tris, 150 mM NaCl, pH 7.4, and 400 μL aliquots were polymerized with 2 units/mL of fish thrombin in tissue culture wells.
Viscoelastic characterization of material scaffolds
The dynamic shear moduli of PA and fibrin gels were measured on a strain-controlled rheometrics fluids spectrometer III (Rheometrics, Piscataway, NJ). A 500-μL sample was polymerized between two steel plates, and the shear modulus G′(ω), which describes elastic resistance, was calculated from the shear stress in phase with a 2% oscillatory (1 rad/s) shear strain. The dynamic shear modulus of adult rat brain was similarly measured. An 8-mm diameter sample was cut using a stainless steel punch and placed between the plates. Short-term G′(ω) was measured by oscillation at 2% strain and the long-term shear modulus G(t) was measured by applying a 10% steady strain and allowing the sample to relax for 30 s.
Fluorescent labeling of adhesive proteins
Fibrinogen and laminin (1 mg/mL) were conjugated to rhodamine-succinimide or fluorescein isothiocyanate (FITC) for fluorescence visualization. Rhodamine-succinimide was added at a ratio of four rhodamine molecules per fibrinogen (pH 8.2). FITC dissolved in dimethyl sulfoxide was added at a ratio of 10 μg per mg of protein in phosphate buffered saline (PBS pH = 7.4) and incubated for 1 h at room temperature. Unreacted rhodamine-succinimide and FITC were removed by dialysis in PBS. Quantification of gel surface fluorescence intensity was performed using NIH Image J software. Five arbitrary fluorescent images of each gel were captured with a Hamamatsu camera (Hamamatsu, Japan) and fluorescence histograms were averaged for statistical analysis.
Cell culture and microscopy
Neuronal and glial cells were derived from prenatal rat embryos and maintained in an incubator at 37°C and 5% CO2. Embryos (E17–E19) were removed by caesarean section from a timed-pregnant Sprague-Dawley rat and the cortices were removed. Tissue was digested in trypsin/DNase at 37°C, centrifuged (1000 g × 5 min), and filtered to derive a cell suspension. For cultures containing both neurons and glial cells, cells were plated directly onto substrates. For rat primary astrocyte cultures, cells were maintained for 14 days in culture with a series of trypsinizations to remove neurons. Cultures used for experiments were >98% astrocytes as assessed by GFAP immunocytochemistry. Cells were grown in an incubator at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (BioWhittaker, East Rutherford, NJ) supplemented with Ham's F12 (Sigma) and 5% fetal bovine serum (Hyclone, Logan, UT) for 7 days followed by an additional 5 days culture in Neurobasal (Gibco, Carlsbad, CA) also supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 50 μg/mL streptomycin, and 50 units/mL penicillin. Astrocytes were plated on 18 mm diameter gels. At 48 h, the cells were examined using a Leica (Wetzlar, Germany) inverted microscope, and images were acquired with a Hamamatsu camera. Neuron and astrocyte cocultures were plated on gels and, at 6 days the cultures were examined. Individual cell morphology was assessed using NIH Image J software.
Immunocytochemistry
Cultures were fixed with 4% paraformaldehyde at 37°C for 30 min. Samples were blocked and permeabilized in 5% bovine serum albumin and 0.2% Triton X-100. The following primary antibodies and stains were used: glial fibrillary acidic protein (GFAP) (1:150, Chemicon, Temecula, CA), βIII-tubulin (1:1000, Chemicon), rhodamine-phalloidin (Molecular Probes, Eugene, OR), and 4′-6-diamidino-2-phenylindole (DAPI) (Sigma). The cell nuclei were counted after separating the red and green channels of the triple-stained images. Cell type was assigned by examining what color stain surrounded the nucleus.
RESULTS
Characterization of protein attachment to gel surface by fluorescence intensity
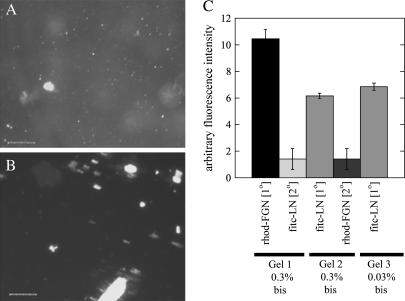
PA gels were prepared as substrates for primary cortical neurons and astrocytes by modifying the method most commonly used to covalently link extracellular matrix ligands to the PA gels (3,21). Acrylic acid N-hydroxy succinimide ester (NHS) monomer incorporated into the polymerizing gel only at the water/toluene interface. The reactive gel surface was covered with a solution of the extracellular matrix protein, which bound the gel by an amide bond. The coupling efficiency was verified by incubating FITC-labeled fibrinogen with PA gels formed with NHS acrylate at their surface (Fig. 1 A). The FITC-fibrinogen was also incubated on plastic as a control for fluorescence (Fig. 1 B). The fluorescence image of the activated gel surface appears uniform compared to plastic typically used in vitro, demonstrating that the density of adhesion sites is without surface irregularities that could influence the structure of adherent cells.
FIGURE 1.
Micrographs and quantification of fluorescence intensity of bound rhodamine and FITC-labeled fibrinogen (fgn) and laminin (LN) to PA gels. (A) Micrograph of PA gels polymerized with N-hydroxy succinimide linker and polystyrene (B) coated with FITC-labeled fgn. PA gel with linker is a uniformly coated matrix. (C) Quantification of total fluorescence intensity of protein coating on PA gel surface shows that attaching 1 mg/mL rhodamine-labeled fgn (1) to the gel saturates its surface binding sites and prevents a second protein, 1 mg/mL FITC-LN, from attaching. As a control, the order of attached proteins was reversed with FITC-LN first and rhodamine-fgn second (2). Also, protein density on gel surface is not related to gel stiffness (3).
To verify that binding an initial protein to the gel surface effectively prevents a second protein from attaching, rhodamine-labeled fibrinogen or FITC-labeled laminin was incubated on an active gel followed by a second incubation with the protein labeled with the other fluorophore. Fluorescence micrographs were captured, and the intensity of each fluorophore was calculated. Fluorescence of the primary protein conjugated to the gel was considerably higher than the secondary protein, showing that reactive groups for ligand attachment were quenched after the initial incubation step (Fig. 1 C, Gel 1). Experiments were repeated using 1 mg/mL FITC-labeled laminin as the primary ligand and as in Gel 1, rhodamine intensity was lower than when fibrinogen was the primary ligand (Fig. 1 C, Gel 2). Therefore, an additional benefit of the PA gel method is that ligand-cell interaction can be studied with few additional adhesion sites created by protein in the serum or released by cells.
Finally, varying the %bis (cross-linker) concentration had no discernible effect on protein conjugation to the gel surface after incubation with equal concentrations of FITC-laminin. Therefore soft and hard gels have an equivalent protein coating (Fig. 1 C, Gel 2 and Gel 3), ensuring that any change in cell behavior on different compliances will be due to mechanical and not chemical stimuli.
Elastic properties of gels and CNS tissues
Viscoelastic measurements using a strain-controlled rheometer verified that shear moduli (G′) of PA gels and fibrin gels increase with cross-linking density or protein concentration, respectively, as has been previously reported (12,22) over a range that spans the elastic moduli reported for brain and spinal cord (17,18,23). Whole brain from adult rat was also measured by rheology and compared with previous reports. Both oscillatory shear and stress relaxation tests were performed to attain short-term and long-term shear modulus values. Short-term (oscillatory) G′(ω) of adult rat brain averaged ∼330 Pa, not far from G′(ω) of the softest PA and fibrin gels, which were 200 Pa and 250 Pa, respectively. Long-term G(t) of rat brain, reported a lower value whose average was 125 Pa, demonstrating the brain's stress relaxation response that has been previously reported (24). Stiffest gels have G′(ω) that are above physiological values for normal brain but similar to those of other tissues and far below that of polystyrene typically used in vitro, with stiff PA and fibrin gels having moduli of 9000 Pa and 2100 Pa, respectively. The results are reported in Table 1.
TABLE 1.
Shear modulus of central nervous system tissue dissected from normal adult rat brain, PA gels, and fibrin gels (mean ± SD), measured by strain oscillation
| Tissue or gel | Mean shear modulus (Pa) |
|---|---|
| Adult rat brain (2% oscillatory strain), G′(ω) | 330 ± 100 |
| Adult rat brain (10 s of constant 10% strain), G(t) | 125 ± 40 |
| 0.01% bis-acrylamide 7.5% acrylamide (soft) gel, G′(ω) | 200 ± 50 |
| 0.3% bis-acrylamide 7.5% acrylamide (hard) gel, G′(ω) | 9000 ± 200 |
| 3 mg/mL salmon fibrin gel, G′(ω) | 250 |
| 18 mg/mL salmon fibrin gel, G′(ω) | 2150 |
Morphology and cytoskeleton of neurons and astrocytes on flexible substrates
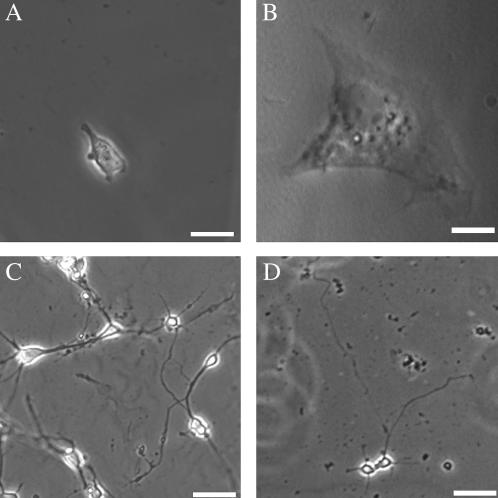
Astrocytes and neurons from dissociated embryonic rat cortices were plated on PA gels and imaged within 7 days. Similar to previous studies of fibroblasts and endothelial cells (3,11,12,25), astrocytes were small and round on soft gels. In comparison, astrocytes on hard gels displayed highly spread morphologies more typical of reactive cultured glia (Fig. 2, A and B). Neurons, in contrast, extended long neurites on both soft and hard substrates, and morphology differences due to compliance change were not obvious in the presence of glia (Fig. 2, C and D).
FIGURE 2.
Phase images of astrocytes on soft (A) and hard (B) LN-coated PA gels 2 days after plating on gels. Astrocytes on soft gels are smaller and less spread than on hard gels. Cultures of dissociated embryonic cortices at 2 days in vitro on soft (C) and hard (D) gels show insignificant differences between neurons on the two gels. Scale bars, 50 μm (A,B) and 25 μm (C,D).
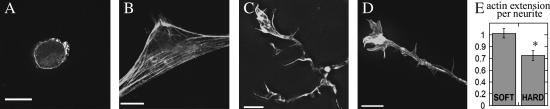
Examination of neuron and astrocyte F-actin structure by rhodamine-phalloidin staining showed differences in cytoskeleton assembly due to substrate compliance. Astrocytes lacked well-articulated F-actin structures on soft gels; phalloidin staining revealed no stress fibers running through the cell body, whereas stress fibers were readily seen for astrocytes grown on hard gels (Fig. 3, A and B). In contrast to the weakly filamentous structure of actin in astrocytes on soft gels, neurons on soft gels had strong F-actin spikes in their growth cones and many actin-rich structures protruding off neurites. The neuronal growth cone appeared branched and filamentous on both soft and hard gels (Fig. 3, C and D). Quantification of F-actin staining (Fig. 3 E) showed that neurons on soft gels have 30% more F-actin structures extending off the length of the neurite, a difference that was statistically significant (t-value = 3.27). Since these filamentous structures eventually result in increased branches and neurites, these results are consistent with previous findings of increased neurite branching on soft substrates (8,10).
FIGURE 3.
Staining for F-actin with phalloidin was used to demonstrate the difference in effect of gel stiffness on actin assembly in both cell types. Astrocytes (A,B) and neurons in mixed culture (C,D) were plated on soft (200 Pa) (A,C) and hard (9 kPa) (B,D) LN-coated PA gels. Actin assembly is disrupted in astrocytes on soft gels as compared to hard, whereas neurons on soft gels have robust actin filaments and enhanced F-actin protrusions (inset). Scale bars, 15 μm (A,B) and 10 μm (C,D).
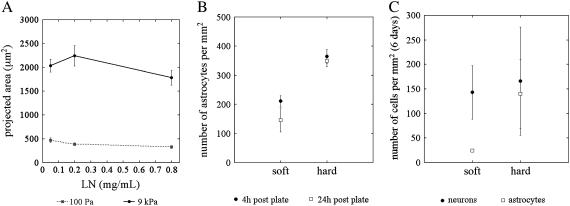
Astrocyte morphology was quantified, and the projected cell area on soft gels was found to be significantly smaller than the area on hard gels as expected from the phase and fluorescent micrographs (Fig. 4 A). There was no statistically significant effect on the projected area of cells bound to either hard or soft gels as the concentration of ligand added to the gel surface was varied from 0.05 mg/mL to 0.2 mg/mL. This result is consistent with the data in Fig. 1, suggesting that the limited number of reactive sites on the gel are mostly saturated with adhesive protein on both hard and soft gels.
FIGURE 4.
The projected cell area on soft gels was smaller than on hard gels (A). There was no statistically significant effect on projected area of cells both to hard and soft gels as the concentration of ligand added to the gel surface was varied from 0.05 mg/mL to 0.2 mg/mL (A). Astrocyte adhesion decreased with gel stiffness, and the number of astrocytes on soft gels was higher at 4 h after plating compared to 24 h after (B). In mixed cultures, astrocyte adhesion increased with shear modulus, but neuronal adhesion was the same for both soft and hard gels (C).
To test for differences in adhesion on hard and soft surfaces, cortical astrocytes were plated at low density (∼3 × 105 cells) on 18 mm gels coated with laminin. At 4 and 24 h, the cells were fixed and stained with DAPI and the cell number was counted for soft and hard PA gels (Fig. 4 B). Astrocyte adhesion decreased with shear modulus of the gels, and the number of astrocytes on soft gels was slightly higher at 4 h after plating compared to gels examined at 24 h, suggesting that cells might detach from soft gels with time. Cells from whole cortical dissociations were plated (1 × 106 cells) on laminin-coated gels and fixed and immunostained with antibodies against the neuron-specific marker βIII-tubulin and the astrocyte-specific GFAP at 6 days after plating. Whereas astrocyte adhesion increased with shear modulus as in the pure cultures, neuronal adhesion was the same for both soft and hard gels (Fig. 4 C).
Evaluation of cocultures of astrocytes and neurons on flexible substrates
Mixed cultures of cortical neurons and astrocytes were imaged 7 days after plating on laminin-coated PA gels. Cultures were immunostained with antibodies against βIII-tubulin and GFAP (Fig. 5, A and B). Neurons grew independently of astrocytes only on soft gels. On hard gels, cells reactive with astrocyte markers were the most abundant cell type, and neurons appeared only to grow over glia. Results show that ∼80% of cells on soft gels were βIII-tubulin positive, whereas on hard gels neurons made up <45% of the population (Fig. 5 C); the remaining cells were nearly all GFAP positive, a significant difference at the 99% confidence level (t-value = 8.02). Fig. 4 shows that astrocyte adhesion was decreased on soft gels, either due to migration off the soft gel or detachment, possibly explaining the lower population of astrocytes on soft gels in the mixed culture.
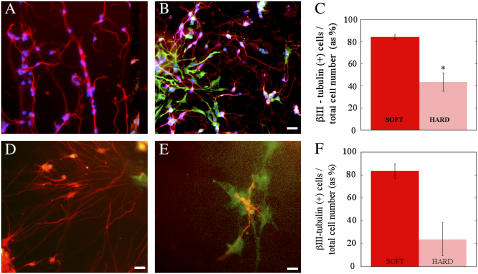
FIGURE 5.
Cultures of dissociated embryonic cortices at 1 week in vitro on soft (A) and hard (B) PA gels and soft (250 Pa) (D) and hard (2.1 kPa) (E) fibrin gels. Neurons were labeled for βIII-tubulin (red) and astrocytes for GFAP (green). All cell nuclei on PA gels were labeled with DAPI (blue). The prevalence of astrocytes is apparent on hard PA gels and to a lesser extent on hard fibrin gels, which are still much softer than the hardest PA gels. The percentage of total cells on soft PA gels (C) and fibrin gels (F) that were neurons (βIII-tubulin positive) was significantly higher than on hard gels (p < 0.01).
To compare the selective growth of neurons on soft PA gels with the response of cells on biologically compatible soft gels, cocultures were also grown on fibrin gels. Similar to the effect of PA gel surfaces, cocultures of neurons and astrocytes on fibrin gels of varying concentration showed a striking difference in the number and type of adherent cells (Fig. 5, D and E). Gels with a fibrinogen concentration of 2 mg/mL had an approximate shear modulus of 250 Pa, whereas increasing the fibrinogen concentration to 18 mg/mL produced a significant increase in the shear modulus to 2100 Pa (Table 1). The number of neurons was determined after 3 days in culture. As confirmed by immunocytochemistry, cortical cell preparations seeded on soft fibrin gels yielded mostly neurons, with ∼80% of total cells staining positive for βIII-tubulin. In contrast, a significantly smaller fraction of cells were neurons on harder fibrin gels at the same time point (Fig. 5 F).
DISCUSSION
By varying the elastic modulus of materials on which CNS cells are grown, we can maximize either neuronal cell survival and extension with minimal attachment of glial cells or else robust growth of astrocytes alongside neurons that are presumably being supported by either the mechanical presence of astrocytes, a soluble factor released by the astrocytes, or a combination of the two.
PA gels are controllable, optically ideal hydrogels, but toxic before polymerization, limiting these polymers to two-dimensional in vitro studies. In contrast, fibrin gels are a natural material that could transition from in vitro studies to in vivo as well as provide three-dimensional matrices for culture studies. Mammalian fibrin has been successfully used as a neuronal substrate in culture (26), and fish fibrin has nearly identical polymerization activity with the added benefit of having been derived from a cold-blooded animal, therefore, potentially lowering the incident of infectious disease transfer (19).
Soft tissues in vertebrates maintain mechanical properties, quantified by an elastic modulus, within a specific range that varies according to tissue type, development, and disease. Brain is a soft tissue and has an elastic modulus ∼10 times softer than liver and nearly 50 times softer than muscle (P. C. Georges, unpublished data; (6)). As with nearly all soft tissues, brain tissue shows stress relaxation, with the shear modulus decreasing to a few hundred Pa in several seconds, consistent with previous reports (24). Historically, most of the focus on brain material properties was for short duration loading, where the brain shows a considerably stiffer compliance. However, for cellular processes such as growth cone advance, the long-term response of brain tissue may be more applicable. Past studies show that neurite motility occurs over the course of seconds to minutes, within the timescale where the brain is considerably softer.
On soft gels astrocytes show fewer stress fibers, consistent with previous findings in fibroblasts where stress fibers fail to form (11). Whether the polymerization machinery of actin is altered or whether lack of focal adhesions to anchor the actin and create stress fibers is the cause is unclear. Like fibroblasts, astrocytes have a large spread area on hard compared to soft substrates, but the stiffness range over which they undergo the transition from round to well spread is lower than that of fibroblasts, beginning below 1 kPa (data not shown) and saturating above 2 kPa on fibrin gels (Fig. 5 E). Fibroblasts would perceive 1 kPa as indistinguishable from 100 Pa, and a soft matrix on which no stress fibers form (10,11) and would see a 2 kPa as an intermediate stiffness. The stiffness sensing of astrocytes has not yet been reported, to our knowledge, and it is noteworthy that astrocytes have dissimilar stiffness sensing from neurons. Neurons are able to polymerize F-actin on soft and hard gels alike, a surprising finding since most cell types have disordered F-actin structures on soft materials. Neurons on the softest gels show increased neurite sprouting, paving the way for an increased number of dendrites and axons and potentially functional connections. The enhanced protrusion of neurites on soft gels together with the increase in branching probability (7) suggests that, despite the similarities between the neuronal growth cone and the lamellapodium of other cell types, the actin-dependent contraction of neurons may not be the motor that drives neurites forward. Previous studies of traction forces exerted by neurons and fibroblasts on PA gels report that whereas fibroblast lamellae can strongly deform gels with elastic moduli near 10,000 Pa, neurons can deform gels only when they are as soft as 50–200 Pa, even though the actin and myosin content of fibroblast lamellae and neuronal growth cones are similar and the traction forces are myosin II dependent (27). The primary force for neurite extension may be due to microtubule or actin dynamics, whereas the contractile forces of the growth cone may serve to guide the neurite and enhance the persistence of its motion.
In this study, neurons grown with astrocytes develop well in both hard and soft cultures. This result does not counter previous findings where neurons have different growth trends on hard materials (8,10)—in this work neurons on hard substrates are making cellular contact with astrocytes. Neurons on soft materials sense a soft substrate of nearly 250 Pa with negligible glial interaction due to a remarkably low number of astrocytes. On hard gels, however, neurons are not purely sensing the kPa matrix they occupy; the inherent mechanical properties of the astrocytes with which they make cell-cell contact must be considered. Furthermore, it is known that astrocytes release soluble factors that support neuronal growth, which could also contribute to neuronal growth on hard gels (1).
The main result of these experiments is the distribution and population sorting of CNS cells that occurs based on the mechanical properties of the substrate. The selectivity of cell adhesion and growth is attributed to external compliance presented to the cells by the extracellular matrix. Neurons grow into hydrogels in injury cavities, whereas astrocytes avoid the same material (13). The results in this study may partly explain how the hydrogel is advantageous postinjury, simultaneously promoting neurite regeneration and suppressing glial ingrowth. In a larger context, this work suggests that the mechanical properties of a biomaterial used for CNS implantation strategies—a largely overlooked characteristic of the implant—may have considerable influence on the form and type of regeneration observed across a CNS material construct.
Acknowledgments
We thank Taylor Cohen of U Penn for providing adult rat brains and Margaret McCormick for her expertise utilizing fibrin gels.
This work was supported by National Science Foundation grant DMR-00-79909, National Institutes of Health grant HL64388, and Department of Defense Small Business Technology Transfer A023-0028.
References
- 1.Fawcett, J. W., and R. A. Asher. 1999. The glial scar and central nervous system repair. Brain Res. Bull. 49:377–391. [DOI] [PubMed] [Google Scholar]
- 2.Horner, P. J., and F. H. Gage. 2000. Regenerating the damaged central nervous system. Nature. 407:963–970. [DOI] [PubMed] [Google Scholar]
- 3.Pelham, R. J. Jr., and Y. Wang. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 94:13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischofs, I. B., and U. S. Schwarz. 2003. Cell organization in soft media due to active mechanosensing. Proc. Natl. Acad. Sci. USA. 100:9274–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janmey, P. A., and D. A. Weitz. 2004. Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem. Sci. 29:364–370. [DOI] [PubMed] [Google Scholar]
- 6.Discher, D. E., P. Janmey, and Y. L. Wang. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science. 310:1139–1143. [DOI] [PubMed] [Google Scholar]
- 7.Engler, A. J., M. A. Griffin, S. Sen, C. G. Bonnemann, H. L. Sweeney, and D. E. Discher. 2004. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanagan, L. A., Y. E. Ju, B. Marg, M. Osterfield, and P. A. Janmey. 2002. Neurite branching on deformable substrates. Neuroreport. 13:2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georges, P. C., and P. A. Janmey. 2005. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 98:1547–1553. [DOI] [PubMed] [Google Scholar]
- 10.Balgude, A. P., X. Yu, A. Szymanski, and R. V. Bellamkonda. 2001. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 22:1077–1084. [DOI] [PubMed] [Google Scholar]
- 11.Engler, A., L. Bacakova, C. Newman, A. Hategan, M. Griffin, and D. Discher. 2004. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 86:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung, T., P. C. Georges, L. A. Flanagan, B. Marg, M. Ortiz, M. Funaki, N. Zahir, W. Ming, V. Weaver, and P. A. Janmey. 2005. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 60:24–34. [DOI] [PubMed] [Google Scholar]
- 13.Woerly, S., V. D. Doan, N. Sosa, J. de Vellis, and A. Espinosa-Jeffrey. 2004. Prevention of gliotic scar formation by NeuroGel allows partial endogenous repair of transected cat spinal cord. J. Neurosci. Res. 75:262–272. [DOI] [PubMed] [Google Scholar]
- 14.Lesny, P., J. De Croos, M. Pradny, J. Vacik, J. Michalek, S. Woerly, and E. Sykova. 2002. Polymer hydrogels usable for nervous tissue repair. J. Chem. Neuroanat. 23:243–247. [DOI] [PubMed] [Google Scholar]
- 15.Loh, N. K., S. Woerly, S. M. Bunt, S. D. Wilton, and A. R. Harvey. 2001. The regrowth of axons within tissue defects in the CNS is promoted by implanted hydrogel matrices that contain BDNF and CNTF producing fibroblasts. Exp. Neurol. 170:72–84. [DOI] [PubMed] [Google Scholar]
- 16.Teng, Y. D., E. B. Lavik, X. Qu, K. I. Park, J. Ourednik, D. Zurakowski, R. Langer, and E. Y. Snyder. 2002. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA. 99:3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirakawa, K., K. Hashizume, and T. Hayashi. 1981. Viscoelastic property of human brain—for the analysis of impact injury. No To Shinkei. 33:1057–1065. [PubMed] [Google Scholar]
- 18.Miller, K., K. Chinzei, G. Orssengo, and P. Bednarz. 2000. Mechanical properties of brain tissue in-vivo: experiment and computer simulation. J. Biomech. 33:1369–1376. [DOI] [PubMed] [Google Scholar]
- 19.Michaud, S. E., L. Z. Wang, N. Korde, R. Bucki, P. K. Randhawa, J. J. Pastore, H. Falet, K. Hoffmeister, R. Kuuse, R. Uibo, J. Herod, E. Sawyer, and P. A. Janmey. 2002. Purification of salmon thrombin and its potential as an alternative to mammalian thrombins in fibrin sealants. Thromb. Res. 107:245–254. [DOI] [PubMed] [Google Scholar]
- 20.Fitch, M. T., C. Doller, C. K. Combs, G. E. Landreth, and J. Silver. 1999. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 19:8182–8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandley, B. K., O. A. Weisz, and R. L. Schnaar. 1987. Cell attachment and long-term growth on derivatizable polyacrylamide surfaces. J. Biol. Chem. 262:6431–6437. [PubMed] [Google Scholar]
- 22.Carr, M. E. Jr., and S. L. Carr. 1995. Fibrin structure and concentration alter clot elastic modulus but do not alter platelet mediated force development. Blood Coagul. Fibrinolysis. 6:79–86. [DOI] [PubMed] [Google Scholar]
- 23.Gefen, A., N. Gefen, Q. Zhu, R. Raghupathi, and S. S. Margulies. 2003. Age-dependent changes in material properties of the brain and braincase of the rat. J. Neurotrauma. 20:1163–1177. [DOI] [PubMed] [Google Scholar]
- 24.Bilston, L. E., Z. Liu, and N. Phan-Thien. 1997. Linear viscoelastic properties of bovine brain tissue in shear. Biorheology. 34:377–385. [DOI] [PubMed] [Google Scholar]
- 25.Dembo, M., and Y. L. Wang. 1999. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 76:2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittier, R., F. Sauthier, J. A. Hubbell, and H. Hall. 2004. Neurite extension and in vitro myelination within three-dimensional modified fibrin matrices. J. Neurobiol. 63:1–14. [DOI] [PubMed] [Google Scholar]
- 27.Bridgman, P. C., S. Dave, C. F. Asnes, A. N. Tullio, and R. S. Adelstein. 2001. Myosin IIB is required for growth cone motility. J. Neurosci. 21:6159–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]