Abstract
Yersinia pestis is transmitted by fleas and causes bubonic plague, characterized by severe local lymphadenitis that progresses rapidly to systemic infection and life-threatening septicemia. Here, we show that although flea-borne transmission usually leads to bubonic plague in mice, it can also lead to primary septicemic plague. However, intradermal injection of Y. pestis, commonly used to mimic transmission by fleabite, leads only to bubonic plague. A Y. pestis strain lacking the plasmid-encoded cell-surface plasminogen activator, which is avirulent by intradermal or s.c. injection, was able to cause fatal primary septicemic plague at low incidence, but not bubonic plague, when transmitted by fleas. The results clarify a long-standing uncertainty about the etiology of primary septicemic plague and support an evolutionary scenario in which plague first emerged as a flea-borne septicemic disease of limited transmissibility. Subsequent acquisition of the plasminogen activator gene by horizontal transfer enabled the bubonic form of disease and increased the potential for epidemic spread.
Keywords: vector, borne disease
The Gram-negative bacterial agent of plague, Yersinia pestis, persists in many wild rodent populations throughout the world and is transmitted by fleas. Susceptible rodent populations are subject to periodic explosive epizootics of plague, and three human plague pandemics have caused millions of deaths (1). Population genetics and comparative genomics analyses indicate that Y. pestis evolved from the closely related food- and water-borne enteric pathogen Yersinia pseudotuberculosis within the last 10,000 years (2, 3). Thus, both the arthropod-borne route of transmission and the increased virulence that distinguish Y. pestis are relatively recent phenomena.
Bubonic plague, the most common form of the disease in both rodents and humans, results when Y. pestis disseminates from a peripheral fleabite site via the lymphatic system to the regional draining lymph nodes. The bacteria multiply and cause acute lymphadenopathy, resulting in the formation of a pathognomonic bubo, an edematous, necrotic, swollen, and painful lymph node (4, 5). Bubonic plague usually progresses rapidly to septicemia, resulting in systemic spread and death caused by severe sepsis. This stage of the disease is referred to as septicemic plague. Whether septicemic plague is always secondary to lymphadenitis is unclear, however. Humans with septicemic plague sometimes have no history of a bubo, and this clinical form has been termed primary septicemic plague (1, 6–8). Hematogenous spread to the lungs leads to pneumonic plague in ≈5% of human cases (9).
Development of disease from peripheral injection routes depends on a plasminogen activator (Pla) encoded by the pla gene located on the 10-kb pPst plasmid that is unique to Y. pestis (10, 11). Typical Pla− Y. pestis strains have greatly reduced virulence when inoculated s.c. but are fully virulent when inoculated directly into the blood stream (12, 13). Pla is a cell-surface protease that induces fibrinolysis and degrades extracellular matrix and basement membranes (14–16). It has been hypothesized that these activities disrupt the host’s ability to contain the bacteria at peripheral infection sites and that Pla is a required virulence factor for flea-borne plague. However, Pla− Y. pestis are occasionally isolated from natural plague foci (17, 18).
In this study, we examined the role of Pla in Y. pestis invasiveness in the natural context of the arthropod-borne route of transmission and determined that two distinct pathologies can ensue from a fleabite: bubonic plague, which depends on Pla, and primary septicemic plague, which does not.
Results
Bubonic or Primary Septicemic Plague Can Follow Transmission by Fleas.
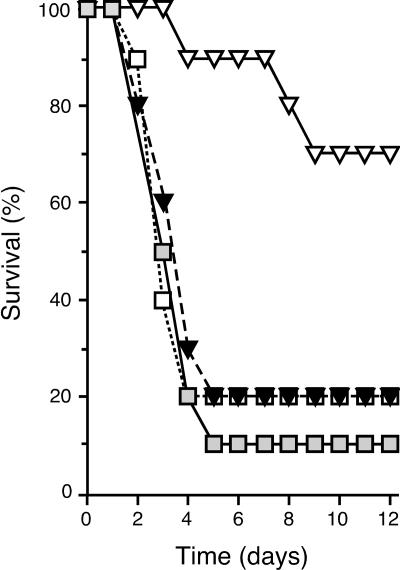
We compared the infectivity and pathogenicity of the wild-type Y. pestis 195/P strain and an isogenic Pla-negative mutant after both artificial transmission by intradermal (ID) needle inoculation and natural transmission by infected Xenopsylla cheopis fleas. Nine of 10 mice injected with ≈100 wild-type Y. pestis developed fatal bubonic plague (Fig. 1). Before the onset of terminal disease, mice developed a limp in the limb closest to the injection site, a sign of localized pain characteristic of bubonic plague lymphadenitis. Eight of 10 mice bitten by fleas infected with wild-type Y. pestis also developed terminal plague (Fig. 1), but unlike the artificial transmission route, flea-borne transmission led to two distinct pathologies. Six of the eight mice developed the severe lymphadenopathy characteristic of bubonic plague (Fig. 2A and D). Two mice, however, developed primary septicemic plague instead of bubonic plague; these mice did not limp, and at the terminal stage of disease their proximal lymph nodes were normal and contained only a few intravascular bacteria (Fig. 2 B and E). Regardless of transmission route or lymph node involvement, terminal disease occurred 2–5 days after challenge, and the degree of sepsis was the same for all mice infected with wild-type Y. pestis (Fig. 3).
Fig. 1.
Effect of the Y. pestis Pla on transmission by fleas. Shown are the incidence and time to terminal disease in mice bitten by fleas infected with Y. pestis wild type (□), Pla− mutant (▿), or complemented mutant (▾) and in mice injected ID with ≈100 Y. pestis wild type (□).
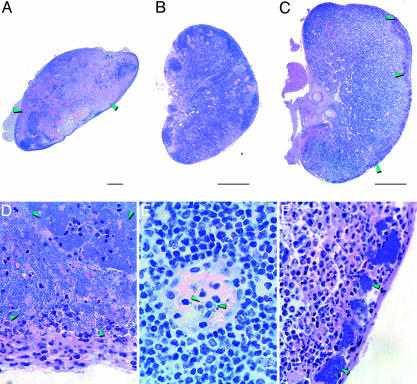
Fig. 2.
Flea-borne transmission of Y. pestis leads to two distinct forms of plague. (A and D) Lymph nodes from mice infected with wild-type Y. pestis showing the severe lymphadenopathy typical of bubonic plague, characterized by destruction of the normal tissue and its replacement with myriad Y. pestis. (B, C, E, and F) Normal-appearing lymph nodes from mice with primary septicemic plague caused by wild-type Y. pestis (B and E) or Pla− Y. pestis (C and F). In primary septicemic plague, extracellular bacteria (green arrowheads) were intravascular (E) or localized to small peripheral clusters and associated with neutrophilic inflammation (F). (Scale bars: 0.5 mm; magnification: D and F, ×600; E, ×1,000.)
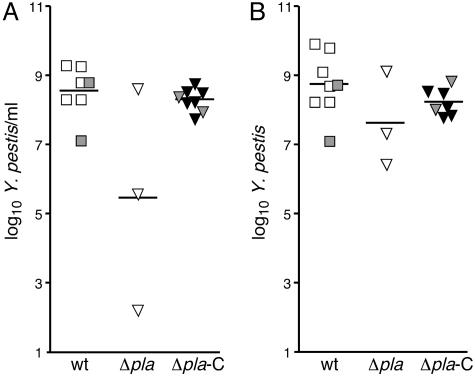
Fig. 3.
Pla− Y. pestis produces systemic infection after flea-borne transmission. Bacterial loads in blood (A) and spleen (B) of individual mice infected with wild-type (squares), Pla− (▵), or complemented Pla− (▴) Y. pestis. Gray symbols indicate Pla+-infected mice with primary septicemic plague. Horizontal bars indicate the mean.
Pla− Y. pestis Causes Primary Septicemic Plague, but Not Bubonic Plague, After Transmission by Fleas.
The results suggested a model in which Y. pestis can disseminate by two distinct routes after transmission by fleabite. One, the well established route via the lymphatic system that leads to bubonic plague, occurred most often (75% of mice); the other led directly to primary septicemic plague (25% of mice). Key support for the model came from results with a Y. pestis Pla− mutant. Consistent with previous reports (12, 13, 19), the LD50 of our Pla− Y. pestis strain was <10 after i.v. inoculation, but ≈106 when injected ID (Table 1). Pla is not required to produce a transmissible infection in X. cheopis fleas, and an uncharacterized isolate of Y. pestis lacking the plasmid that encodes Pla was shown to be infectious by X. cheopis fleabite (20, 21). Therefore, it was possible to evaluate the effect of Pla on Y. pestis infectivity after flea-borne transmission. Three of 10 mice challenged by fleas infected with Pla− Y. pestis developed terminal plague (Fig. 1 and Table 1). The flea-bitten mice received an inoculum well below the LD50 of Pla− Y. pestis by ID inoculation because the transmission efficiency of X. cheopis is low. Only about half of individual bites result in successful transmission, with a median of ≈80 Y. pestis transmitted per bite (22). Notably, none of the sick mice infected by fleabite with the Pla− mutant had the bubonic form of the disease. They did not limp, and the lymph nodes proximal to the fleabite site were either uninfected or contained only small discrete clumps of bacteria in the marginal sinus (Fig. 2 C and F) at the terminal stage of the disease, when the peripheral blood, spleen, and liver were all heavily infected (Figs. 3 and 4). Thus, although the Pla− Y. pestis could disseminate to the regional lymph nodes after fleabite transmission, as others have observed after s.c. inoculation (13), the infection did not progress to lymphadenitis and bubonic plague. Complementation of the Y. pestis Pla− mutant with a wild-type copy of the Y. pestis pla gene restored full virulence after either ID inoculation or fleabite (Figs. 1 and 3 and Table 1). Mice infected with the complemented mutant or the wild-type strain also had the same incidence of the bubonic (75%) and nonbubonic (25%) forms of plague after equivalent fleabite challenge.
Table 1.
Effect of transmission route on virulence of Pla− Y. pestis
| Y. pestis strain | Transmission route |
||
|---|---|---|---|
| i.v. | ID | Fleabite | |
| Wild type | <10 | 46 | 8/10 |
| Δpla | <10 | 1.7 × 106 | 3/10 |
| Δpla (complemented) | <10 | 17 | 8/10 |
LD50 values are given for i.v. and ID injection and disease incidence for fleabite transmission (no. of mice that developed terminal disease/no. of mice challenged).
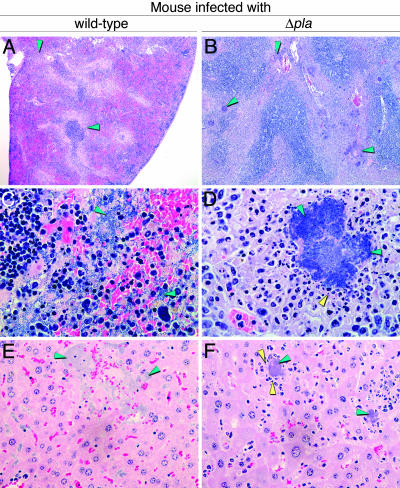
Fig. 4.
Differential histopathology of primary septicemic plague produced by Pla+ and Pla− Y. pestis after fleabite transmission. Extracellular bacteria (green arrowheads) are widely dispersed in spleen (A and C) and liver (E) infected with wild-type Y. pestis, but are contained in granulomatous-like lesions surrounded by degrading host cells (yellow arrowheads) in spleen (B and D) and liver (F) infected with Pla− Y. pestis. (Magnification: A and B, ×4; C, ×600; D–F, ×400.)
Although the incidence of primary septicemic plague was similar after fleabite transmission regardless of the presence or absence of Pla, certain Pla-related differences were observed in pathogenesis. The time to terminal disease was significantly longer for mice infected with Pla− Y. pestis (P < 0.0065 by log rank test; Fig. 1). Pla activity, essential to overcome the lymphatic tissue barrier in bubonic plague, may also shorten the retention time in the splenic filter in septicemic plague. Even though the Pla+ and Pla− strains achieved equivalent bacterial loads in the spleen at the terminal stage of disease (Fig. 3), many Pla− Y. pestis, unlike wild type, were contained within developing granulomas (Fig. 4). In addition, the terminal bacteremia level in two of the three mice infected with Pla− Y. pestis was lower than in mice infected with the Pla+ strain (Fig. 3).
Effect of Flea Salivary Gland Extract on Y. pestis Infectivity After ID Injection.
The saliva of blood-feeding arthropods contains pharmacologically active compounds that interfere with normal hemostatic and innate immune responses of the mammalian host (23–25). For example, flea saliva contains the anticoagulant apyrase, an enzyme that inhibits platelet and neutrophil aggregation (26). To investigate whether flea saliva enhances entry into the peripheral vasculature, additional groups of 10 mice were injected ID with Y. pestis in PBS with or without added flea salivary gland extract. When the inocula contained salivary gland extract, 8 of 9 mice infected with wild-type Y. pestis and 1 of 10 mice infected with the pla mutant developed bubonic plague. With the same inocula suspended in PBS only, 9 of 10 mice infected with wild-type Y. pestis and 2 of 10 mice infected with the pla mutant mice developed bubonic plague. Thus, the presence of salivary gland extract did not enhance the infectivity or affect the dissemination route of ID-injected bacteria.
Discussion
Primary and Secondary Septicemic Plague.
The etiology of human septicemic plague has been a matter of controversy. Up to 30% of human plague patients develop septicemia with no clinical history of a bubo (6–8), but the pathophysiological relationship between primary septicemic and bubonic plague has remained uncertain. Most early workers distinguished only bubonic and pneumonic forms of the disease, concluding that septicemic plague was always a secondary sequela of frank or subclinical bubonic plague (6, 7, 9, 27). Others recognized primary septicemic plague, but assumed that the bacteria passed through the lymph nodes before entering the blood (28). Our results provide evidence that primary septicemic plague is a distinct clinical entity and indicate that Y. pestis can disseminate from a fleabite site and produce fatal sepsis by different routes: through the lymphatic system, directly through the circulatory system, or both.
ID injection of wild-type Y. pestis always led to bubonic plague, characterized by acute lymphadenopathy and destruction of normal lymph node tissue (Fig. 2). This picture was usually seen after fleabite transmission also; however, 25% of mice developed septicemic plague without lymphadenitis. At the terminal stage of systemic sepsis, their lymph nodes were normal and contained only intravascular bacteria, indicative of hematogenous rather than lymphatic origin (5). Nevertheless, given the rapid dissemination from the fleabite site and fulminant development of sepsis, it is difficult to rule out the possibility that septicemia sometimes develops by rapid transit through the draining lymph nodes without lymphadenitis. It is not clear why this would happen only after flea-borne transmission and not ID injection, however. Perhaps the strongest evidence for primary septicemic plague came from results with the Pla− Y. pestis strain. Like the wild-type parent strain, this mutant could disseminate to the draining lymph nodes after either ID injection or fleabite transmission (Fig. 2). Whereas dissemination to the lymph node regularly led to bubonic plague with wild-type Y. pestis, it never did so for the Pla− strain. On the other hand, primary septicemic plague occurred in 25–30% of mice infected with either Pla+ or Pla− bacteria, but only after fleabite transmission. These findings suggest that primary septicemic plague results from invasion of blood vessels at the dermal infection site, because it seems unlikely that the mode of transmission to the dermis would determine systemic spread from the regional lymph nodes.
Importance of the Host–Vector Interface in Flea-Borne Plague.
The factors specific to the microenvironment of the fleabite that are required to establish primary septicemic plague are unknown, but underscore the importance of studying Y. pestis pathogenesis in the natural context of arthropod-borne transmission. Fleas initially deposit Y. pestis into dermal tissue, and not s.c., because the infection that develops at the bite site is ID (Fig. 5A); at any rate, the flea mouthparts are not long enough to penetrate through the dermis (Fig. 5B). Fleas, like mosquitoes, are thought to cannulate small blood vessels when they feed (29, 30). However, normal blood feeding is blocked in fleas that transmit plague, and blocked fleas repeatedly probe the dermis in their futile attempts to obtain a blood meal. Low numbers of Y. pestis are transmitted during these feeding attempts (22), and it is possible that some bacteria are injected directly into the dermal blood vessels as well as extravascularly. Some support for direct i.v. transmission by fleas came from the fact that ID injection in the presence of X. cheopis salivary gland extract did not result in primary septicemic plague, suggesting that anticoagulant or other components in flea saliva do not enhance indirect invasion of the bloodstream from the extravascular dermal tissue.
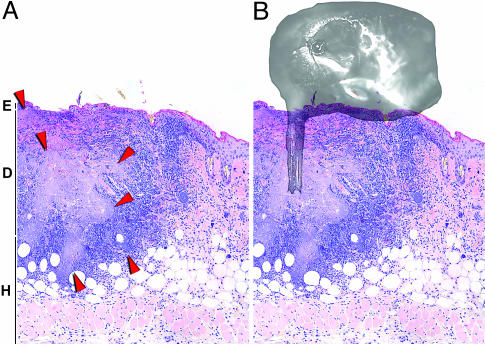
Fig. 5.
Fleas transmit Y. pestis ID. (A) Skin section of a fleabite site 3 days after infection with wild-type Y. pestis. A large bacterial mass (light purple area surrounded by red arrowheads) extends from the middle of the dermis to the hypodermal border. E, epidermis; D, dermis; H, hypodermis. (B) Apposition of the head of an X. cheopis flea with its mouthparts centered over the infecting mass of bacteria. (Magnification: ×100.)
The Possible Sequential Emergence of Primary Septicemic and Bubonic Forms of Plague.
Arthropod-borne transmission evolved relatively recently in the genus Yersinia (2, 3). As for many bacterial pathogens, genetic accretion by horizontal transfer from unrelated bacteria, selective gene loss, and changes in regulatory pathways appear to have been critical to the evolution of Y. pestis (31–33). Acquisition by the ancestral Y. pseudotuberculosis strain of a new plasmid encoding a phospholipase D, which enhanced survival in the flea gut, and adaptation of preexisting biofilm-forming capability to block the flea foregut were likely precipitating events in the transition to flea-borne transmission (34, 35). Our results indicate that such an intermediate progenitor clone could persist in rodent–flea transmission cycles, and Pla− Y. pestis have been isolated from plague foci worldwide (17, 18). Subsequent horizontal transfer of the plasmid encoding Pla would have enabled the bubonic form of plague, substantially increasing disease incidence after fleabite (Fig. 1 and Table 1). The increased invasiveness brought by Pla may have also enhanced transmissibility to the vector, because unlike the Pla− strain, Pla+ Y. pestis produced bacteremia that was consistently higher than the threshold level required to reliably infect fleas (22) (Fig. 3). The basic reproductive rate of an arthropod-borne agent, an estimate of epidemic potential, is directly related to both the proportion of vector bites that lead to systemic infection and the duration of a threshold bacteremia level (22, 36). Thus, although not essential for flea-borne transmission or virulence, Pla may have enabled the epidemic amplification and spread in susceptible host populations that characterizes modern plague.
Methods
Bacterial Strains.
A Pla− strain of the fully virulent Y. pestis 195/P strain in which a 1,052-bp segment containing the pla promoter and coding sequence was replaced with the aph kanamycin resistance cassette was produced by mobilization of the suicide plasmid pCVD442 (37) containing aph from pUC4K flanked by 500 bp of Y. pestis sequence upstream and downstream of the intended deletion. Allelic exchange was verified by PCR analysis and loss of fibrinolytic activity (20). The Δpla strain was complemented by electrotransformation with the high copy-number pUC18 vector containing a wild-type copy of the pla gene cloned from Y. pestis 195/P.
Infections.
X. cheopis fleas were infected by allowing them to feed on blood containing ≈5 × 108 Y. pestis per ml, using an artificial feeding system, and maintained at 21°C and 75% relative humidity as described (17). For flea transmission experiments, a mesh-covered capsule containing 24–57 infected X. cheopis was applied to a shaved area on the dorsolateral surface of restrained 8-to 10-wk-old female RML BALB/c mice. After 60 min, the fleas were removed, and the number of infectious (blocked) fleas that had bitten each mouse was determined (38). All mice received 12 or fewer infectious fleabites in one to three sequential challenges (Table 2).
Table 2.
Summary of fleabite challenges
| Mouse | Mice bitten by fleas infected with: |
|||||
|---|---|---|---|---|---|---|
| Wild type |
Δpla |
Δpla (complemented) |
||||
| Infective fleabites | Outcome | Infective fleabites | Outcome | Infective fleabites | Outcome | |
| 1 | 1 | B | 6 | S | 3 | B |
| 2 | 2 | B | 6 | S | 5 | B |
| 3 | 2 | B | 3 | S | 7 | B |
| 4 | 6 | B | 3 | — | 8 | B |
| 5 | 9 | B | 4 | — | 8 | B |
| 6 | 10 | B | 5 | — | 12 | B |
| 7 | 3 | S | 5 | — | 6 | S |
| 8 | 10 | S | 6 | — | 11 | S |
| 9 | 6 | — | 8 | — | 8 | — |
| 10 | 7 | — | 9 | — | 9 | — |
| Median | 6 | 5.5 | 8 | |||
Shown is the cumulative number of bites from infective fleas, which had the characteristic bacterial blockage of the proventricular valve in the flea foregut that is prerequisite for efficient transmission (see refs. 4 and 38). The median number of infective bites received by each group was not significantly different (P > 0.6 by Mann–Whitney test). B, bubonic plague; S, primary septicemic plague; —, no disease. Disease type and outcome were significantly different for the Δpla group compared with the other two (P < 0.05 by χ2 test).
For ID or i.v. injections, Y. pestis strains were cultured in LB at 28°C, quantified by direct count in a Petroff-Hausser bacterial counting chamber, and diluted in PBS. LD50 values were calculated by the Reed-Muench equation (39), using mortality data from groups of five mice injected with 10 to 107 bacteria in 30 μl (ID injection) or 100 μl (i.v. injection) of PBS. Other groups of 10 mice were injected ID with ≈100 Y. pestis in 50 μl of PBS or with 50 wild-type or 103 Pla− Y. pestis in the presence or absence of a sonicate of dissected X. cheopis salivary glands in 50 μl of PBS (four gland equivalents per injection).
Bacteriology and Histopathology.
Mice were killed upon signs of terminal plague, and the quantity of Y. pestis in heart blood and spleen was determined by colony-forming unit count (5). Inguinal and axillary lymph nodes, a portion of the spleen and liver, and skin biopsies were formalin-fixed. Hematoxylin/eosin-stained sections of the paraffin-embedded tissues were examined microscopically.
Acknowledgments
We thank T. Schwan, F. Gherardini, N. Lemaître, and D. Erickson for critical reading of the manuscript. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and the Ellison Medical Foundation (New Scholars Award in Global Infectious Diseases to B.J.H.).
Abbreviations
- Pla
plasminogen activator
- ID
intradermal.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Perry R. D., Fetherston J. D. Clin. Microbiol. Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achtman M., Zurth K., Morelli G., Torrea G., Guiyoule A., Carniel E. Proc. Natl. Acad. Sci. USA. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achtman M., Morelli G., Zhu P., Wirth T., Diehl I., Kusecek B., Vogler A. J., Wagner D. M., Allender C. J., Easterday W. R., et al. Proc. Natl. Acad. Sci. USA. 2004;101:17837–17842. doi: 10.1073/pnas.0408026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollitzer R. Plague. Geneva: W.H.O; 1954. [Google Scholar]
- 5.Sebbane F., Gardner D., Long D., Gowen B. B., Hinnebusch B. J. Am. J. Pathol. 2005;166:1427–1439. doi: 10.1016/S0002-9440(10)62360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flexner S. Am. J. Med. Sci. 1901;122:396–416. [Google Scholar]
- 7.Crowell B. C. Philippine J. Sci. 1915;10:249–307. [Google Scholar]
- 8.Hull H. F., Montes J. M., Mann J. M. J. Infect. Dis. 1987;155:113–118. doi: 10.1093/infdis/155.1.113. [DOI] [PubMed] [Google Scholar]
- 9.Butler T. Plague and Other Yersinia Infections. New York: Plenum; 1983. [Google Scholar]
- 10.Sodeinde O. A., Goguen J. D. Infect. Immun. 1989;57:1517–1523. doi: 10.1128/iai.57.5.1517-1523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonough K. A., Falkow S. Mol. Microbiol. 1989;3:767–775. doi: 10.1111/j.1365-2958.1989.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 12.Sodeinde O. A., Subrahmanyam Y. V., Stark K., Quan T., Bao Y., Goguen J. D. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 13.Welkos S. L., Friedlander A. M., Davis K. J. Microb. Pathog. 1997;23:211–223. doi: 10.1006/mpat.1997.0154. [DOI] [PubMed] [Google Scholar]
- 14.Goguen J. D., Bugge T., Degen J. L. Methods. 2000;21:179–183. doi: 10.1006/meth.2000.0989. [DOI] [PubMed] [Google Scholar]
- 15.Lähteenmäki K., Kuusela P., Korhonen T. K. Methods. 2000;21:125–132. doi: 10.1006/meth.2000.0983. [DOI] [PubMed] [Google Scholar]
- 16.Kukkonen M., Lähteenmäki K., Suomalainen M., Kalkkinen N., Emödy L., Lång H., Korhonen T. K. Mol. Microbiol. 2001;40:1097–1111. doi: 10.1046/j.1365-2958.2001.02451.x. [DOI] [PubMed] [Google Scholar]
- 17.Filippov A. A., Solodovnikov N. S., Kookleva L. M., Protsenko O. A. FEMS Microbiol. Lett. 1990;55:45–48. doi: 10.1016/0378-1097(90)90165-m. [DOI] [PubMed] [Google Scholar]
- 18.Cavalcanti Y. V., Leal N. C., de Almeida A. M. Lett. Appl. Microbiol. 2002;19:915–918. doi: 10.1046/j.1472-765x.2002.01226.x. [DOI] [PubMed] [Google Scholar]
- 19.Brubaker R. R., Beesley E. D., Surgalla M. J. Science. 1965;149:422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- 20.Hinnebusch B. J., Fischer E. R., Schwan T. G. J. Infect. Dis. 1998;178:1406–1415. doi: 10.1086/314456. [DOI] [PubMed] [Google Scholar]
- 21.de Almeida A. M. P., Alves L. C., Amaral R. L. G., França W. G. B., Leal N. C. Parasitol. Res. 2003;89:159–162. doi: 10.1007/s00436-002-0731-3. [DOI] [PubMed] [Google Scholar]
- 22.Lorange E. A., Race B. L., Sebbane F., Hinnebusch B. J. J. Infect. Dis. 2005;191:1907–1912. doi: 10.1086/429931. [DOI] [PubMed] [Google Scholar]
- 23.Titus R. G., Ribeiro J. M. C. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 24.Zeidner N. S., Schneider B. S., Nuncio M. S., Gern L., Piesman J. J. Parasitol. 2002;88:1276–1278. doi: 10.1645/0022-3395(2002)088[1276:COBSWT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Ramamoorthi N., Narasimhan S., Pal U., Bao F., Yang X. F., Fish D., Anguita J., Norgard M. V., Kantor F. S., Anderson J. F., et al. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro J. M. C., Vaughan J. A., Azad A. F. Comp. Biochem. Physiol. B. 1990;95:215–219. doi: 10.1016/0305-0491(90)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Wu L.-T., Chun J. W. H., Pollitzer R., Wu C. Y. Shanghai: Mercury; 1936. Plague: A Manual for Medical and Public Health Workers. [Google Scholar]
- 28.Barker L. F. Am. J. Med. Sci. 1901;122:377–395. [Google Scholar]
- 29.Lavoipierre M. M. J., Hamachi M. Nature. 1961;192:998–999. [Google Scholar]
- 30.Deoras P. J., Prasad R. S. Ind. J. Med. Res. 1967;55:1041–1050. [PubMed] [Google Scholar]
- 31.Groisman E. A., Casadesús J. Mol. Microbiol. 2005;56:1–7. doi: 10.1111/j.1365-2958.2005.04564.x. [DOI] [PubMed] [Google Scholar]
- 32.Carniel E. Adv. Exp. Med. Biol. 2003;529:3–12. doi: 10.1007/0-306-48416-1_1. [DOI] [PubMed] [Google Scholar]
- 33.Chain P. S. G., Carniel E., Larimer F. W., Lamerdin J., Stoutland P. O., Regala W. M., Georgescu A. M., Vergez L. M., Land M. L., Motin V. L., et al. Proc. Natl. Acad. Sci. USA. 2004;101:13826–13831. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinnebusch B. J., Rudolph A. E., Cherepanov P., Dixon J. E., Schwan T. G., Forsberg #x00C5;. Science. 2002;296:733–735. doi: 10.1126/science.1069972. [DOI] [PubMed] [Google Scholar]
- 35.Jarrett C. O., Deak E., Isherwood K. E., Oyston P. C., Fischer E. R., Whitney A. R., Kobayashi S. D., DeLeo F. R., Hinnebusch B. J. J. Infect. Dis. 2004;190:783–792. doi: 10.1086/422695. [DOI] [PubMed] [Google Scholar]
- 36.Anderson R. M., May R. M. Population Biology of Infectious Diseases. New York: Springer; 1982. [Google Scholar]
- 37.Donnenberg M. S., Kaper J. B. Infect. Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinnebusch B. J., Perry R. D., Schwan T. G. Science. 1996;273:367–370. doi: 10.1126/science.273.5273.367. [DOI] [PubMed] [Google Scholar]
- 39.Reed L. J., Muench H. A. Am. J. Hyg. 1938;27:493–497. [Google Scholar]