Abstract
Despite evidence that stressful experience can exacerbate the symptoms of asthma, little is known about the biological mechanisms through which this occurs. This study examined whether life stress reduces expression of the genes coding for the glucocorticoid receptor and the β2-adrenergic receptor. A total of 77 children were enrolled in the study (59% male; mean age, 13.5 years). Thirty-nine of them were physician-diagnosed with asthma, and 38 were healthy. After an in-depth interview regarding stressful experiences, leukocytes were collected through antecubital venipuncture, and real-time RT-PCR was used to quantify mRNA. Chronic stress was associated with reduced expression of mRNA for the β2-adrenergic receptor among children with asthma. In the sample of healthy children, however, the direction of this effect was reversed. The occurrence of a major life event in the 6 months before the study was not sufficient to influence patterns of gene expression. When such events occurred in the context of a chronic stressor, however, their association with patterns of gene expression was accentuated. Children with asthma who simultaneously experienced acute and chronic stress exhibited a 5.5-fold reduction in glucocorticoid receptor mRNA and a 9.5-fold reduction in β2-adrenergic receptor mRNA relative to children with asthma without comparable stressor exposure. These findings suggest that stressful experience diminishes expression of the glucocorticoid and β2-adrenergic receptor genes in children with asthma. To the extent that it diminishes sensitivity to the antiinflammatory properties of glucocorticoids or the bronchodilatory properties of β-agonists, this process could explain the increased asthma morbidity associated with stress.
Mounting evidence indicates that stressful experience can exacerbate the symptoms of asthma in childhood (1–5). For example, in an 18-month prospective study of children with ongoing asthma, severe life events increased the risk of an attack by nearly 2-fold. The impact of life events was accentuated when they occurred in the context of a chronic stressor. Children exposed to high levels of acute and chronic stress showed a 3-fold increase in risk for an attack in the 2 weeks that followed (6, 7).
Despite this robust pattern of findings, little is known about the responsible underlying mechanisms (1). Stressful experiences typically activate the hypothalamic–pituitary–adrenocortical and sympathetic–adrenal–medullary axes, leading to increased secretion of the hormones cortisol, epinephrine, and norepinephrine. Although these molecules are commonly thought to be mediators of stress-related disease (8), the role they play in linking stress and asthma is less clear. High levels of cortisol diminish inflammation in the airways and the periphery; similarly, β-adrenergic agonists such as epinephrine are potent bronchodilators. These observations suggest the paradoxical conclusion that stressful experiences should ameliorate rather than exacerbate symptoms of asthma (9, 10). The clinical data gathered to date, however, are inconsistent with this conclusion. Stressful experiences are associated with worse asthma outcomes (1, 2).
In an effort to resolve this paradox and identify potential underlying mechanisms, we have proposed an alternative hypothesis linking stressful experience with asthma morbidity (9). It maintains that stressors foster persistent secretion of the hormonal products of the hypothalamic–pituitary–adrenocortical and sympathetic–adrenal–medullary axes. With continued exposure to elevated concentrations of these molecules, target tissues undergo a compensatory down-regulation of glucocorticoid receptors (GRs) and adrenergic receptors. This process diminishes sensitivity to the antiinflammatory properties of glucocorticoids and the bronchodilatory properties of β-agonists and by doing so helps foster persistent inflammation and airway constriction upon exposure to asthma triggers.
The current article examines the first step in this chain of events in a community sample of children with asthma. We conducted detailed interviews regarding acute and chronic forms of life stress and collected leukocytes to quantify the expression of mRNA for the GR and the β2-adrenergic receptor (β2AR). The primary hypotheses were (i) that both acute and chronic stressors would be associated with reduced expression of receptor mRNA and (ii) that the impact on gene expression would be most marked when an acute event was superimposed on existing chronic stressors.
Results
Preliminary Analyses.
Table 1 describes the characteristics of the sample. Children with asthma and healthy children were similar in terms of age, ethnic/racial background, and family income (P values > 0.41). The groups differed with respect to gender distribution, however, with a greater proportion of males in the asthma cohort (χ2 = 4.78, P = 0.04). The groups had similar body-mass indices (P > 0.95), although as expected, children with asthma had lower forced expiratory volume (FEV1) percentiles (t(75) = 2.27, P < 0.03).
Table 1.
Characteristics of the sample
| Demographics and medical characteristics | Asthma(n = 39) | Healthy(n = 38) |
|---|---|---|
| Demographics | ||
| Age, years | 13.4 ± 2.7 | 13.5 ± 2.1 |
| Sex, no. of males* | 28 (71%) | 18 (47%) |
| Descent (European) | 24 (61.5%) | 25 (65.8%) |
| Descent (Asian) | 9 (23.1%) | 8 (21.1%) |
| Annual income (more than $50,000) | 24 (61.5%) | 25 (65.8%) |
| Medical characteristics | ||
| Body-mass index, kg/m2 | 20.8 ± 3.2 | 20.8 ± 3.6 |
| FEV1 (% of predicted)* | 94.0 ± 13.5 | 102.9 ± 10.1 |
| Inhaled corticosteroids (>3 times per week)* | 12 (15.6%) | — |
| Bronchodilator (>3 times per week)* | 11 (14.3%) | — |
| Leukotriene antagonist (>3 times per week)* | 2 (2.6%) | — |
*Asthma and healthy groups differ at P < 0.05.
The study’s major predictor and outcome variables are summarized in Table 2. Children with asthma and healthy children were rated as having similar levels of chronic stress in the family, home-life, academic, and friendship domains (P values > 0.41). They also had experienced similar numbers of major life events in the 3- and 6-month periods before enrollment (P values > 0.39). Significant differences in mRNA for the GR and β2AR were evident. Because each unit difference in relative quantity indicates a 2-fold disparity in gene expression, children with asthma expressed more than four times as much β2AR mRNA (t(75) = 4.32, P < 0.001) and nearly twice as much GR mRNA as medically healthy children (t(75) = 1.97, P < 0.05).
Table 2.
Summary of predictors and outcomes
| Predictors and outcomes | Asthma (n = 39) | Healthy (n = 38) |
|---|---|---|
| Stress ratings | ||
| Chronic stress | ||
| Family relationships (1–5 rating) | 2.2 ± 0.6 | 2.4 ± 1.0 |
| Home life (1–5 rating) | 2.5 ± 0.7 | 2.5 ± 1.0 |
| Academic (1–5 rating) | 2.2 ± 0.8 | 2.2 ± 0.9 |
| Friendships (1–5 rating) | 2.1 ± 0.8 | 2.2 ± 0.7 |
| Life event | ||
| Past 3 months | 7 (17.9%) | 5 (13.2%) |
| Past 6 months | 8 (20.5%) | 7 (18.4%) |
| mRNA expression | ||
| GR (RQ, log-2)* | 4.7 ± 1.5 | 3.8 ± 1.5 |
| β2AR (RQ, log-2)* | 6.8 ± 2.5 | 4.5 ± 2.2 |
RQ, relative quantity of mRNA.
*Asthma and healthy groups differ at P < 0.05.
To identify potential confounders, correlations were computed between children’s demographic and biomedical characteristics and outcomes. Relative quantities of mRNA were similar across demographic categories (P values > 0.21) but, as would be expected, were consistently related to FEV1 percentile and to use of inhaled corticosteroids and bronchodilators (P values < 0.05). Specifically, children who regularly used bronchodilators showed higher quantities of GR and β2AR mRNA than children who did not (P values < 0.05). A similar pattern was evident for use of inhaled corticosteroids (P values < 0.05). (These differences reflect the fact that medications were used only by asthmatic children, who as a group showed increased expression of both transcripts relative to healthy children. Among asthmatic children there was a small but nonsignificant decrease in mRNA among children taking corticosteroids and bronchodilators.) Variables reflecting body-mass index and use of leukotriene modifiers were unrelated to β2AR and GR. On the basis of the results of these analyses, we included FEV1 percentile and use of corticosteroids and bronchodilators as covariates in all subsequent analyses.
Role of Chronic Stressors.
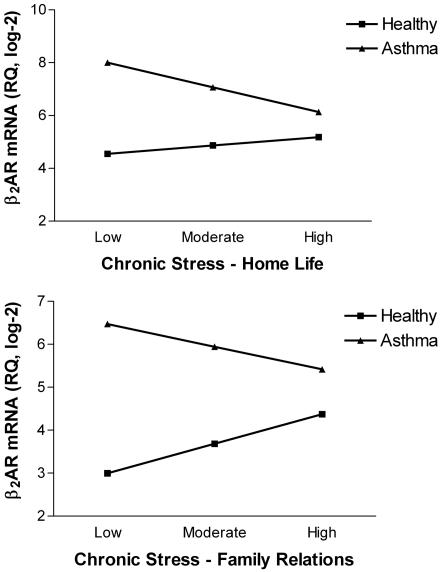
Regression analyses indicated that relationships between chronic stress and receptor mRNA differed for children with asthma vs. healthy children. Fig. 1 illustrates these findings graphically. For the β2AR, there was a significant interaction between medical-group status and chronic stress in the home (B = 1.59, SE = 0.76, P < 0.04, ΔR2 = 0.05). In children with asthma, quantities of β2AR mRNA declined markedly as home life became more stressful, whereas in healthy children, receptor expression increased in a modest fashion with chronic stress (Fig. 1 Upper).
Fig. 1.
Relationship between chronic stress and β2AR expression differs for children with asthma vs. healthy children. Among children with asthma, quantities of β2AR mRNA decline as home life (Upper) and family relationships (Lower) contain higher levels of chronic stress. The reverse pattern is evident in healthy children. As home life (Upper) and family relationships (Lower) worsen, expression of the β2AR gene increases. RQ, relative quantity of mRNA.
A similar pattern emerged for chronic family stress (for the interaction, B = 1.56, SE = 0.76, P < 0.05, ΔR2 = 0.04). Among children with asthma, poor-quality family relationships were associated with reduced β2AR mRNA, whereas among healthy children, these variables were positively related (Fig. 1 Lower). In both sets of analyses, children with asthma had greater quantities of β2AR mRNA than healthy children (P values < 0.001), but there were no main effects of chronic stress (P values > 0.35). The lack of main effects is not surprising in light of the robust interactions, which show that the direction of the stress–mRNA association differs by medical-group status.
Despite the association between chronic stress and β2AR, a similar pattern did not emerge in analyses of GR. Ratings of chronic stress in the family and home-life domains were unrelated to GR mRNA, regardless of whether they were considered as main effects or in conjunction with medical-group status (P values > 0.11). Moreover, associations between chronic stress ratings in the academic and friendship domains and receptor mRNA were nonsignificant (P values > 0.10).
Role of Major Life Events.
ANOVAs did not reveal associations between major life events and β2AR or GR mRNA. This was the case regardless of whether the events had occurred in a 3- or 6-month window and whether their presence was considered in conjunction with the children’s medical status (P values > 0.12). Thus, the simple presence of a major life event was not sufficient to influence expression patterns of the genes encoding β2AR and GR.
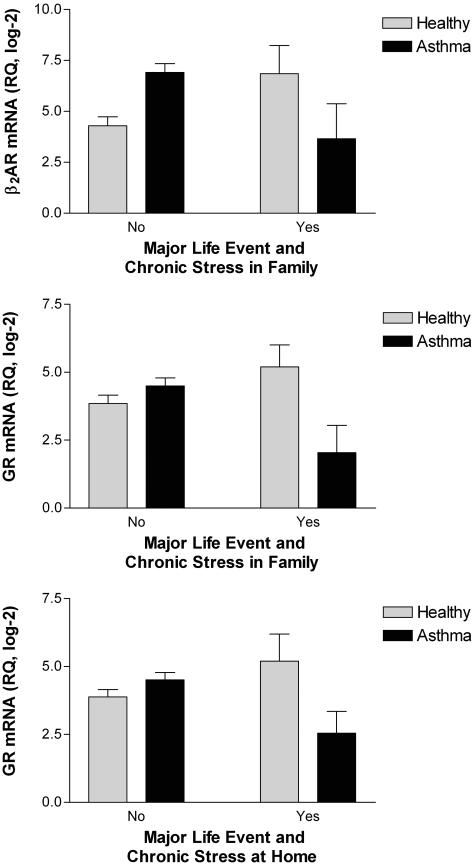
When acute events were considered in the context of chronic stress, however, the magnitude of their association with β2AR and GR mRNA was accentuated. Fig. 2 illustrates these findings graphically for events that occurred within 3 months of enrollment. For β2AR, there was a significant interaction between medical-group status and double exposure, defined as the occurrence of a major event and ongoing chronic stress in the family (F(1,69) = 6.40, P < 0.02). Among children with asthma, the combination of acute and chronic stress was associated with a marked reduction in β2AR mRNA (Fig. 2 Top). This effect was quite large in magnitude, amounting to a 9.5-fold disparity in gene expression between children with asthma who had and did not have double exposure. Among the healthy children, this pattern was reversed; the combination of acute and chronic stress was associated with a 5.5-fold increase in β2AR mRNA.
Fig. 2.
When acute events occur in the midst of ongoing chronic stress, their impact on expression of β2AR and GR mRNA is accentuated. Children with asthma experience a 9.5-fold reduction in β2AR mRNA (Top) and a 5.5-fold reduction in GR mRNA (Middle) when they are simultaneously exposed to a major life event and chronic family stress. In healthy children, this pattern is reversed (all panels). A marked decline in GR mRNA also occurs in children with asthma who experience a major life event at the same time as chronic stress in the home (Bottom). Data are plotted as mean ± SEM. RQ, relative quantity of mRNA.
A similar pattern of findings emerged in analyses of the GR. There was a significant interaction between medical-group status and double exposure for major acute events and chronic family stress (F(1,69) = 7.36, P < 0.01). Fig. 2 (Middle) displays this finding graphically. Children with asthma experienced a 5.5-fold reduction in GR mRNA when they were exposed to acute and chronic stressors simultaneously. In healthy children, this pattern was reversed and weaker; double exposure was associated with a 2.5-fold increase in transcript. When chronic stress in the home-life domain was considered, a significant interaction of the same nature emerged (F(1,69) = 5.11, P < 0.03). Fig. 2 (Bottom) shows that double exposure resulted in a 4-fold decline in GR mRNA in children with asthma and a 2.5-fold increase in expression of this gene among medically healthy children.
To confirm these findings, analyses were repeated with a 6-month window for major life events. The pattern of results was nearly identical, with significant medical-group status by double-exposure interactions, both for β2AR and for GR (F(1,69) values ≥ 4.83, P values < 0.04). In all cases, double exposure was associated with reduced mRNA in children with asthma and increased mRNA in healthy children (data not shown). However, the magnitude of these effects was somewhat smaller than that with a 3-month window, with disparities in the 2.5- to 4.0-fold range for children with asthma and in the 1.6- to 6.0-fold range for healthy children.
Specificity of Findings.
The final analyses examined whether our findings were specific to β2AR and GR mRNA. We repeated all of the major analyses in the paper, this time attempting to predict expression of 18S, the housekeeping gene quantified as part of the PCR procedure. Stressful experiences were unrelated to quantities of 18S mRNA, regardless of whether they were acute or chronic in nature (P values > 0.18). These findings suggest that life stress is likely to have a specific impact on GR and β2AR, rather than globally decreasing mRNA expression in leukocytes.
Discussion
These findings demonstrate a relationship between stressful life experiences and the expression of mRNA for β2AR and GR. To the extent that their home and family lives were marked by chronic stress, children with asthma showed a decline in mRNA for the β2AR. The occurrence of major life events in the 6 months before the study was not sufficient to influence patterns of gene expression. When such events occurred in the context of a chronic stressor, however, their association with gene expression was greatly accentuated. Children with asthma exposed to a major life event in the midst of chronic family stress showed a 9.5-fold reduction in β2AR mRNA and a 5.5-fold reduction in GR mRNA relative to children with asthma without comparable stressor exposure. Similar findings were obtained for chronic stress in the home; when coupled with a major life event, it was associated with a 4-fold reduction in GR mRNA.
How might these disparities explain the excess asthma morbidity associated with stressful experience? β2AR and GR are the starting points for a number of signaling pathways that regulate airway responsiveness and inflammation. On airway smooth muscle cells, β2AR activation facilitates bronchial relaxation. On T lymphocytes, β2AR and GR play key roles in regulating expression of IL-4, IL-5, and IL-13 after allergen exposure. Mast cells also constitutively express these receptors, and ligation can inhibit release of histamine and other allergic mediators as well as diminish recruitment and activation of eosinophils (11–13). Thus, to the extent that the observed disparities in mRNA are manifest in diminished cellular expression of β2AR and GR, children with asthma could be vulnerable to airway inflammation and constriction after exposure to allergic triggers. Apart from these direct influences on airway and immune function, stress-related down-regulation of these receptor systems could diminish sensitivity to β-agonist and glucocorticoid medications, which are central to effective clinical management of asthma (9). Indeed, resistance to glucocorticoid medication is associated with increased morbidity and mortality in patients with acute respiratory distress (14).
These observations underscore the potentially detrimental consequences of abrasive family relationships and unstable home environments for children with asthma. In this regard, they converge with the findings of several recent clinical studies, which have shown that parental stress is prospectively associated with wheezing in infancy (15) and that the emergence of childhood asthma is more common in families with parenting difficulties (5, 16). Moreover, children whose caregivers report high levels of stress and have difficulties parenting are at greatest risk for developing asthma (17). There is also mounting evidence that stressors outside the immediate family are important; exposure to community violence is associated with greater expression of symptoms in children with asthma (4). Collectively, these findings suggest that in children and adolescents with asthma, the quality of home life and family relationships are important determinants of health and well-being and appear to have stronger effects than other life domains, such as academics and peer relationships.
The study also found that asthmatic and healthy children differentially regulate leukocyte gene-expression programs. Children with asthma expressed greater quantities of β2AR and GR mRNA than healthy children. These findings are consistent with research showing that stable asthma is marked by elevated GR density on lymphocytes (18, 19) and increased β2AR on lymphocytes and lung tissue (20, 21). The mechanism(s) underlying this phenomenon are unclear. One possibility is that by expressing greater numbers of receptors, especially those for glucocorticoids, asthmatic patients are better able to regulate cytokine responses to allergic stimuli. A related possibility is that higher receptor numbers help compensate for any resistance to glucocorticoids or bronchodilators that has arisen with long-term exposure to medications that target these systems. Future research will be needed to evaluate these hypotheses, however, because several teams have found similar patterns of receptor expression on the lymphocytes of asthma patients and healthy controls (22, 23). Apart from overall disparities in the quantity of mRNA, our data suggest that stressors differentially regulate gene-expression programs in healthy and asthmatic children. Although stressful experience was associated with down-regulation of mRNA in children with asthma, we found that in healthy children, it exhibited the opposite pattern of association in some cases. These patterns were found only for family stress and only with β2AR mRNA, such that ongoing family stress was related to greater β2AR mRNA among healthy children, and this association was magnified by the occurrence of a major life event. This pattern of findings was unexpected. In healthy adults facing chronic stressors, β2AR receptor density is reduced (24), similar to our asthma patients. Future studies will need to identify the mechanisms underlying this phenomenon in healthy children, to better understand how stressful experiences, and the hormonal cascades they set into motion, alter programs of gene expression in children with asthma vs. healthy children.
There are a number of limitations to this study that should be noted. First, its cross-sectional design precludes inferences about the direction of causality. Although we were able to rule out several alternative explanations for the findings, including demographics, pulmonary function, and medication usage, it is possible that unmeasured variables spuriously inflated the associations between stressful experience and mRNA for β2AR and GR. It is also possible that the direction of causality goes from gene expression to stressful experience, although it is difficult to conceive of how this would operate because the tissue we studied was leukocytes (and not the central nervous system). Second, leukocytes were not collected in a fashion that enabled us to identify which cell(s)’ genetic programs were altered through stressful experience. The patterns we found could have arisen in granulocytes, monocytes, lymphocytes, or some combination of these cell classes. Further research is needed to clarify this issue. Meanwhile, it is important to remember that the β2AR and GR are expressed on most granulocytes, monocytes, and lymphocytes and that each of these cell types participates in one or more phases of the allergic response that gives rise to asthma symptoms (14, 25, 26). Moreover, the relative balance of cell types was unrelated to life stress in this sample, indicating that our findings do not simply reflect stress-related disparities in leukocyte trafficking through the circulation. Lastly, the protocol assessed mRNA in leukocytes collected in peripheral blood. Cells residing in the airways or other lymphoid compartments may have provided a better indication of in vivo circumstances. However, more invasive measures of this nature would have been difficult to obtain in children who were not receiving any obvious clinical benefit for participating. Nevertheless, the next wave of studies will need to evaluate the generality of our findings and determine whether they extend to other tissues involved in asthma pathogenesis. It also will be a high priority to conduct protein-expression and receptor-binding experiments to determine whether the mRNA disparities are reflected in receptor number and function.
Despite these limitations, this study offers insights into the mechanisms through which stressful experience exacerbates the symptoms of asthma. Previous research has found that in children and adolescents with asthma, stressors are associated with higher levels of IgE, greater lymphocyte proliferative responses to allergic triggers, and heightened in vitro production of T helper 2 cytokines such as IL-4 and IL-5 (27–29). Moreover, patients with asthma have been shown to respond to allergen challenges with greater numbers of eosinophils and heightened production of IL-5 during the period surrounding a naturalistic life stressor (30). Our findings extend these observations in two key ways. First, they suggest that specific features of a stressful experience govern whether it relates to biological outcomes. Stressors that are chronic in nature and involve abrasive family relationships or an unstable home environment appear to be most detrimental. Acute stressors occurring in isolation do not influence gene expression, but when they arise in the midst of a chronic stressor, the strength of their association with outcomes is markedly accentuated. Second, our findings extend previous research by demonstrating that stressful experiences are associated with alterations at the level of gene expression and result in lower quantities of mRNA for receptor systems that regulate airway responsiveness and inflammation. With further mechanistic research of this nature, scientists and clinicians will be able to better understand the biology underlying long-held clinical wisdom that stressful experiences contribute to asthma morbidity and mortality.
Methods
Patients.
The sample consisted of 39 children with asthma and 38 medically healthy children. They were recruited from the Vancouver, BC community through advertisements in physicians’ offices, newspapers and magazines, and community settings. Children were eligible for the study if (i) they were between the ages of 9 and 18, (ii) they were fluent in the English language, and (iii) they had been free of upper-respiratory illness for the past 4 weeks. To be included in the asthma group, children were required to have a physician diagnosis of asthma and be free of other chronic medical illness. Healthy children were required to have a history without chronic medical and psychiatric illness. Information regarding children’s medical history was gathered from parents.
Procedures.
Eligible children visited our research center accompanied by a parent. After the study procedures had been explained in detail, written consent was obtained from the parent, and assent was obtained from the child. The child was then taken into a separate room, and a local anesthetic cream (EMLA; AstraZeneca, Wilmington, DE) was applied to the child’s arm in preparation for venipuncture. Children were then interviewed in depth regarding life stress (see below). Pulmonary function testing was performed by using spirometry. Next, the child was seated in a comfortable chair, and blood was collected through antecubital venipuncture. The protocol was approved by the Research Ethics Board of the University of British Columbia.
Life-Stress Interview.
To assess patients’ exposure to stressful experiences, we administered the UCLA Life Stress Interview, Child Version (31). This semistructured interview covers acute and chronic forms of stress over the past 6 months. It focuses on stress in multiple life domains, including family relationships, friendships, school, and home life. (Family relationships and home life are separate domains of chronic stress. The former is concerned with the quality of interpersonal relationships among family members; the latter focuses on work stress in the parents, the financial situation of the family, and the quality of the neighborhood environment.) In each domain, a trained interviewer asked a series of open-ended questions and used the information gathered to rate the level of chronic, ongoing stress. Ratings range from 1 to 5, with higher numbers reflecting more severe and persistent difficulties. The interview also yielded information regarding the occurrence of acute stressors, which in this context are defined as specific events with a discrete onset and offset. To judge the objective impact of an acute event, our research team made a consensus rating after it had been briefed on event details by the primary interviewer. Impact ratings range from 1 (no long-term impact) to 5 (severe long-term impact). Importantly, consensus ratings explicitly consider the context in which an event has occurred. For example, if a child’s grandfather experienced a myocardial infarction, the impact rating would depend on factors such as how close their relationship was, whether the child visited him in the hospital, and whether the child had previous experience coping with serious family illnesses. Following convention, we consider acute events rated >3 (moderate long-term impact) to be major life events. This interview has been used successfully in children as young as 8, and there is evidence to support its reliability and validity. For example, children and adolescents with high stress ratings are prone to the onset of a depressive episode (32–34).
GRs and β2ARs.
Leukocyte expression of GR and β2AR mRNA was quantified through real-time RT-PCR. Blood (2.5 ml) was collected through antecubital venipuncture into PAXgene Blood RNA tubes (Pre-Analytix, Hombrechtikon, Switzerland). Total RNA was then extracted by using PAXgene Blood RNA Kits (Pre-Analytix). RT-PCRs were carried out on an Applied Biosystems Prism 7000 Sequence Detection System, by using a one-step assay based on 5′ nuclease activity of 6-carboxyfluorescein-labeled TaqMan probes (TaqMan One-Step RT-PCR; Applied Biosystems). For β2AR, a commercially available assay system was used (TaqMan Gene Expression Assay #Hs00240532; Applied Biosystems) with primers and probes based on consensus sequences (National Center for Biotechnology Information RefSeq NM_000682). Analyses of GR focused on the dominant receptor isoform GR-α, which transduces antiinflammatory signals of corticosteroids. To quantify expression of GR-α mRNA, we developed a TaqMan assay in collaboration with Applied Biosystems. The primer sequences were AGTGGTTGAAAATCTCCTTAACTATTGCT (forward) and GGTATCTGATTGGTGATGATTTCAGCTA (reverse) and based on established sequences (RefSeq NM_000176) for GR-α. For both assays, the thermal cycling protocol included 30 min of reverse transcription at 48°C, followed by 10 min of AmpliTaq activation at 95°C and 40 cycles of amplification consisting of 15 s of denaturing at 95°C and 60 s of annealing/extension at 60°C. Threshold cycle (CT) numbers were established by using sds 1.1 rq software (Applied Biosystems). As an internal control, levels of 18S mRNA were quantified in parallel with target genes (TaqMan Gene Expression Assay #Hs99999901; Applied Biosystems). The data were normalized by using the ΔCT method (ΔCT = CT target gene − CT internal control). Results are expressed as relative quantities of each target gene, calculated by subtracting each patient’s ΔCT value from the highest ΔCT value in the distribution. Thus, higher relative quantities are indicative of greater expression of the GR and β2AR genes. Each unit difference in relative quantity indicates a 2-fold difference in gene expression, given that during each amplification cycle, there is a doubling of gene product.
Potential Confounders.
We measured a number of processes that could provide alternative explanations for relationships between stressors and biological outcomes. Information regarding demographic characteristics of the child and his/her family was collected during interviews with the parent. Pulmonary function was evaluated through spirometry, and FEV1 values were derived. FEV1 values were calculated as a percentage of predicted values, based on child age, gender, ethnicity, and height. Measures were taken at least 4 h after the last use of a short-acting bronchodilator and followed the protocols of large, multisite clinical asthma trials (35). To evaluate the impact of medication, parents were asked to bring all of their child’s medications to the research center. Names and dosages were recorded directly from the label, and the number of days each medication was taken in the last 2 weeks was ascertained from the parent and child. Two variables were created from these data to use in statistical analyses. One reflected the total number of doses that were administered in the past 2 weeks, and the other reflected the number of days on which the medication was used. Because identical results emerged with both variables, the latter was used in the final round of statistical analyses.
Statistical Analyses.
Three major hypotheses were tested in the statistical analyses. To examine the impact of chronic stressors on GR and β2AR expression, we computed a series of hierarchical multiple regression equations. Relative quantities of each transcript were predicted from three blocks of variables entered consecutively. The blocks consisted of (i) demographic and biomedical variables that represented potential confounds, (ii) indicators of medical-group status (asthma vs. healthy) and chronic stress, and (iii) a product term representing the interaction of the latter factors. When a statistically significant interaction emerged, it was interpreted according to guidelines outlined by Aiken and West (36). The relative quantity of mRNA was estimated at low, moderate, and high levels of chronic stress, and these values were plotted separately for children with asthma vs. healthy children. Following convention, low chronic stress was defined as 1 SD below the sample mean, moderate stress as the mean value, and high chronic stress as 1 SD above the mean. To examine the impact of acute events on GR and β2AR expression, a two-way ANOVA was performed. Medical-group status (asthma vs. healthy) and major life event (absent vs. present) served as the independent variables, and potential demographic and biomedical confounds were modeled as covariates. Lastly, to examine whether the impact of acute events was accentuated by chronic stress, we created a double-exposure variable, defined as having a major event in the past 3 months and a chronic stress rating of moderate (i.e., 3) or greater. A two-way ANOVA was then performed, with medical-group status (asthma vs. healthy) and double exposure (absent vs. present) as independent variables, and potential confounds were modeled as covariates. For all statistical analyses, α was set to 0.05, and two-tailed t tests of significance were performed. Values are expressed as mean ± SD unless otherwise noted.
Acknowledgments
We thank Dr. Steve Cole for helping us design and analyze the real-time RT-PCR experiments. This work was supported by the Michael Smith Foundation for Health Research; the Canadian Institutes of Health Research; the William T. Grant Foundation, and the British Columbia Ministry of Child and Family Development through the Human Early Learning Partnership.
Abbreviations
- β2AR
β2-adrenergic receptor
- FEV1
forced expiratory volume
- GR
glucocorticoid receptor.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wright R. J., Rodriguez M., Cohen S. Thorax. 1998;53:1066–1074. doi: 10.1136/thx.53.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomberg G. R., Chen E. Immunol. Allergy Clin. N. Am. 2005;25:83–105. doi: 10.1016/j.iac.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Busse W. W., Kiecolt-Glaser J. K., Coe C., Martin R. J., Weiss S. T., Parker S. R. Am. J. Respir. Crit. Care. Med. 1995;151:249–252. doi: 10.1164/ajrccm.151.1.7812562. [DOI] [PubMed] [Google Scholar]
- 4.Wright R. J., Mitchell H., Visness C. M., Cohen S., Stout J., Evans R., Gold D. R. Am. J. Public Health. 2004;94:625–632. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klinnert M. D., Nelson H. S., Price M. R., Adinoff A. D., Leung D. Y., Mrazek D. A. Pediatrics. 2001;108:e69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- 6.Sandberg S., Paton J. Y., Ahola S., McCann D. C., McGuinness D., Hillary C. R. Lancet. 2000;356:982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- 7.Sandberg S., Jarvenpaa S., Penttinen A., Paton J. Y., McCann D. C. Thorax. 2004;59:1046–1051. doi: 10.1136/thx.2004.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen B. S. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 9.Miller G. E., Cohen S., Ritchey A. K. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S., Rodriguez M. In: Asthma and Respiratory Infections. Skoner D. P., editor. New York: Dekker; 2001. pp. 193–208. [Google Scholar]
- 11.Heijink I. H., van den Berge M., Vellenga E., de Monchy J. G., Postma D. S., Kauffman H. F. Clin. Exp. Allergy. 2004;34:1356–1363. doi: 10.1111/j.1365-2222.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson M. J. Allergy Clin. Immunol. 2002;110:S282–S290. doi: 10.1067/mai.2002.129430. [DOI] [PubMed] [Google Scholar]
- 13.Umland S. P., Schleimer R. P., Johnston S. L. Pulm. Pharmacol. Ther. 2002;15:35–50. doi: 10.1006/pupt.2001.0312. [DOI] [PubMed] [Google Scholar]
- 14.Meduri G. U., Yates C. R. Ann. N.Y. Acad. Sci. 2004;1024:24–53. doi: 10.1196/annals.1321.004. [DOI] [PubMed] [Google Scholar]
- 15.Wright R. J., Cohen S., Carey V., Weiss S. T., Gold D. R. Am. J. Respir. Crit. Care Med. 2002;165:358–365. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- 16.Mrazek D. A., Klinnert M., Mrazek P. J., Brower A., McCormick D., Rubin B., Ikle D., Kastner W., Larsen G., Harbeck R., et al. Pediatr. Pulmonol. 1999;27:85–94. doi: 10.1002/(sici)1099-0496(199902)27:2<85::aid-ppul4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Klinnert M. D., Mrazek P. J., Mrazek D. A. Psychiatry. 1994;57:51–61. doi: 10.1080/00332747.1994.11024668. [DOI] [PubMed] [Google Scholar]
- 18.Torrego A., Pujols L., Roca-Ferrer J., Mullol J., Xaubet A., Picado C. Am. J. Respir. Crit. Care Med. 2004;170:420–425. doi: 10.1164/rccm.200308-1143OC. [DOI] [PubMed] [Google Scholar]
- 19.Vachier I., Roux S., Chanez P., Loubatiere J., Terouanne B., Nicolas J. C., Godard P. Clin. Exp. Immunol. 1996;103:311–315. doi: 10.1046/j.1365-2249.1996.d01-628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai T. R., Zhou D., Aubert J. D., Lizee G., Hayashi S., Bondy G. P. Am. J. Respir. Cell Mol. Biol. 1993;8:325–333. doi: 10.1165/ajrcmb/8.3.325. [DOI] [PubMed] [Google Scholar]
- 21.Gao H., Xue Q., Lin Y., Wang L., Zhu G., Zhao Q., Chen Y. Chin. Med. J. 2001;114:1317–1319. [PubMed] [Google Scholar]
- 22.Gagliardo R., Chanez P., Vignola A. M., Bousquet J., Vachier I., Godard P., Bonsignore G., Demoly P., Mathieu M. Am. J. Respir. Crit. Care Med. 2000;162:7–13. doi: 10.1164/ajrccm.162.1.9911032. [DOI] [PubMed] [Google Scholar]
- 23.Newnham D. M., Coutie W. J., McFarlane L. C., Lipworth B. J. Eur. J. Clin. Pharmacol. 1993;45:535–538. doi: 10.1007/BF00315310. [DOI] [PubMed] [Google Scholar]
- 24.Mills P. J., Adler K. A., Dimsdale J. E., Perez C. J., Ziegler M. G., Ancoli-Israel S., Patterson T. L., Grant I. Am. J. Geriatr. Psychiatry. 2004;12:281–286. [PubMed] [Google Scholar]
- 25.Blalock J. E. Immunol. Today. 1994;15:504–511. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 26.Busse W. W., Lemanske R. F. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 27.Chen E., Fisher E. B., Jr, Bacharier L. B., Strunk R. C. Psychosom. Med. 2003;65:984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- 28.Kang D., Coe C., McCarthy D. O., Jarjour N. N., Kelly E. A., Rodriguez R. R., Busse W. W. J. Interferon Cytokine Res. 1997;17:481–487. doi: 10.1089/jir.1997.17.481. [DOI] [PubMed] [Google Scholar]
- 29.Wright R. J., Finn P., Contreras J. P., Cohen S., Wright R. O., Staudenmayer J., Wand M., Perkins D., Weiss S. T., Gold D. R. J. Allergy Clin. Immunol. 2004;113:1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Liu L. Y., Coe C. L., Swenson C. A., Kelly E. A., Kita H., Busse W. W. Am. J. Respir. Crit. Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 31.Hammen C. J. Abnorm. Psychol. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 32.Hammen C., Adrian C., Hiroto D. Br. J. Clin. Psychol. 1988;27:37–46. doi: 10.1111/j.2044-8260.1988.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph K. D., Hammen C. Child Dev. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- 34.Adrian C., Hammen C. J. Consult. Clin. Psychol. 1993;61:354–359. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- 35.Childhood Asthma Management Program Research Group Control. Clin. Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 36.Aiken L. S., West S. G. Multiple Regression: Testing and Interpreting Interactions. London: Sage Publications; 1991. [Google Scholar]