Summary
Therapies that target signaling pathways critical to the pathogenesis and progression of squamous cell carcinoma of the head and neck (HNSCC) are needed. One such target, phosphatidylinositol 3-kinase, and its downstream target serine/threonine kinase, Akt, are up-regulated in HNSCC. Targeted therapy could consist of inhibitors of these kinases or, alternatively, of inhibitors of the pathways that they regulate. To explore the effect of Akt inhibition on the growth and survival of HNSCC tumors, we evaluated the effect of a novel Akt inhibitor, KP372-1, on the growth, survival, and sensitivity to anoikis of HNSCC cell lines in culture. Using Western blotting of head and neck cancer cell lines and squamous mucosa and carcinoma specimens, we found that Akt was highly phosphorylated in head and neck cancer cell lines and human head and neck squamous carcinoma specimens. Treatment of HNSCC cell lines with KP372-1 blocked the activation of Akt, inhibited head and neck cancer cell proliferation, and induced apoptosis and anoikis in several HNSCC cell lines. Furthermore, KP372-1 decreased the phosphorylation of the S6 ribosomal (Ser240/244) protein, which is a downstream target of Akt. Taken together, these findings indicate that KP372-1 may be a useful therapeutic agent for HNSCC and should be further evaluated in preclinical models of HNSCC.
Keywords: Akt, anoikis, apoptosis, KP372-1
The abbreviations used are: EGF, epidermal growth factor; GSK-3, glycogen synthase kinase-3; HNSCC, squamous cell carcinomas of the head and neck; MTT, thiazolyl blue tetrazolium bromide; p, phosphorylated; PARP, poly (ADP-ribose) polymerase; PI-3K, phosphatidylinositol 3-kinase; SCC, squamous cell carcinoma
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) is one of the leading causes of cancer deaths worldwide [1]. Despite improvements in surgery, radiotherapy, and conventional chemotherapy, patients at high risk for tumor recurrence, according to clinicopathologic criteria, frequently develop locoregional recurrences, leading to 5-year survival rates that remain at only 30–50% [2]. Although nodal metastases and extension of the tumor beyond the lymph node capsule have long been known as the most predictive factors for regional recurrence and death from HNSCC, little is known about the biologic basis of tumor progression of HNSCC [3]. Therefore, there is a great need to identify the signaling pathways important in the development and progression of HNSCC.
The phosphatidylinositol-3 kinase (PI-3K)/Akt signaling pathway has been shown to mediate tumor cell proliferation and survival and to be up-regulated in a number of tumor types, including HNSCC [4,5,6,7]. Others have shown that higher levels of Akt expression contribute to malignancy and antiapoptotic activity in squamous cell carcinoma (SCC) of oral squamous epithelium [8]. The Akt pathway has also been shown to be important in mediating signals that lead to anchorage-independent survival, and inhibition of the PI-3K/Akt has been shown in other cell types to lead to anchorage-independent cell death, or anoikis [9,10]. Our studies in which oral cavity cancer cells were selected for anchorage-independent survival support the findings of Li et al. [11] that selection for resistance to anoikis leads to aggressive tumor growth and decreased animal survival in an orthotopic model of tongue cancer in nude mice. Given the association between anoikis resistance and the tumor progression of oral SCC and the associations between anoikis resistance and the PI-3K/Akt pathway, we evaluated the effects of a specific Akt inhibitor, KP372-1 (molecular weight 224.2; QLT Inc., Vancouver, BC, Canada), on the inhibition of PI-3K/Akt pathways biochemically and on cell proliferation, apoptosis, and anoikis in head and neck cancer cell lines.
Materials and methods
Cell cultures and reagents
The Tu167, Tu212, Tu159, LN212, UMSCC1, and MDA1986 HNSCC cell lines were obtained from Dr. Gary Clayman at The University of Texas M. D. Anderson Cancer Center Head and Neck Laboratory. All cell lines were maintained in Dulbecco’s modified Eagle’s/F-12 medium supplemented with 10% fetal calf serum. For the selection of anoikis-resistant cells, 2 × 106 Tu167 cells were detached from a tissue-culture flask by treatment with 0.25% trypsin-0.1% ethylenediaminetetraacetic acid solution and then grown in a 15-ml conical tube (Falcon; Becton-Dickinson, Franklin Lakes, NJ) with a vented cap (Biocoat; Becton-Dickinson, Bedford, MA) that was placed on a rotating wheel for 72 h in a humidified incubator at 37°C with 5% CO2. These cells were then plated and grown to confluence under adherent conditions before they were expanded. To generate the JMAR cell line from the Tu167 cell line, this cycle was repeated six times. Limiting dilution cloning of the Tu167 and JMAR cell lines was performed, yielding the cell lines Tu167c2, JMARc39, and JMARc42 [12]. In addition, DM12, a clone of Tu167, was selected for its ability to grow in soft agar.
The following antibodies, reagents, and materials were used: phospho (p) Akt/Ser473, pAkt/Thr308, total Akt, p70S6 kinase, S6 ribosomal protein, pGSK-β antibodies, and an Akt kinase assay kit (all from Cell Signaling Technology, Beverly, MA); anti-actin (Sigma, St. Louis, MO); anti-mouse and anti-rabbit horseradish-peroxidase conjugate (Amersham, Piscataway, NJ); poly (ADP-ribose) polymerase and p85 (Upstate Biotechnology, Lake Placid, NY); and Bax (Santa Cruz Biotechnology, Santa Cruz, CA).
Compounds
KP372-1 (Fig. 1) was synthesized by QLT Inc.; it is a mixture of two isomers present in approximately equal amounts. A stock solution of KP372-1 for enzyme or cellular assays was prepared in dimethyl sulfoxide and then diluted in the medium. The final concentration of dimethyl sulfoxide in the incubation mixture did not exceed 0.1% v/v.
Figure 1.
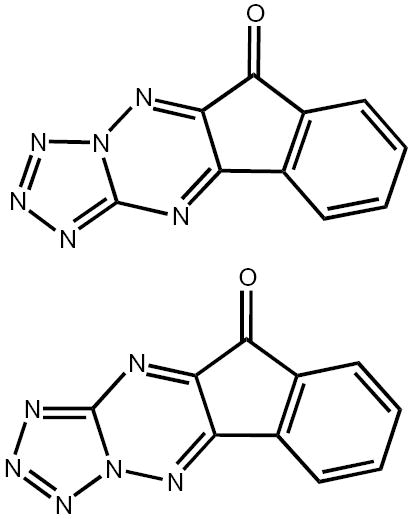
Molecular structure of KP372-1, a mixture of two isomers.
Cell extracts and Western blotting
Cells were lysed in Nonidet P-40 lysis buffer (50 mM Tris HCl [pH 8.0], 137 mM sodium chloride, 10% glycerol, 1% Nonidet P-40, 50 mM sodium fluoride, 10 mM β-glycerol phosphate) containing 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, and 10 ng/ml aprotonin. Cell extracts were separated on a 10% sodium dodecyl sulfate–polyacrylamide electrophoretic gel and transferred to nitrocellulose membranes, which were blocked with bovine serum albumin or fat-free milk and probed with the appropriate antibodies using electrochemiluminescence- or alkaline phosphatase-based color methods.
Tissue samples and Western blotting
Human tissue samples were obtained from the M. D. Anderson head and neck tissue bank with the approval of our institutional review board. Specimens from patients who had undergone surgery had been snap frozen in liquid nitrogen and stored at −80°C.
Thawed tissue samples were homogenized in Triton X-100 lysis buffer (20 mM HEPES, 150 mM NaCl, 1% Triton X-100, 0.1% deoxycholate, 2 mM ethylenediaminetetraacetic acid, 2 mM sodium vanadate, and protease inhibitor cocktail), and equal amounts of protein were analyzed by Western blotting. β-Actin was used as a loading control.
Apoptosis assay
Cells were washed with phosphate-buffered saline and resuspended in 50 μg/ml propidium iodide in 0.1% sodium citrate with 0.1% Triton X-100 for 20 min at 4°C. Cells were then analyzed by flow cytometry, and the sub-G0/G1 fraction was used as a measure of the apoptotic cells.
Cell proliferation
Thiazolyl blue tetrazolium bromide (MTT) assays were carried out as follows: tissue-cultured cells were trypsinized, counted, and diluted to the appropriate concentration. For MTT assays measuring growth only, cells were diluted to 1000 cells per 200 μl, and 200 μl of this cell suspension was added to each well of a 96-well plate. For MTT assays involving treatment with compounds, cells were diluted to 1000 cells per 100 μl of the complete medium, and 100 μl was added to each well of a 96-well plate.
The following day, 100 μl of medium containing the desired concentration of KP372-1 was added to the appropriate wells. The cells were then kept at 37°C in 5% CO2 for the desired length of time. At this point, 10 μl of a 5 mg/ml stock solution of MTT in water was added to each well, and the plates were returned to the 37°C incubator for 2 h. Afterward, the supernatant was aspirated from each well, and 200 μl of dimethyl sulfoxide was added to each well. The plates were then shaken for 5 min, and the optical density was measured at 570 nm using a spectrophotometer.
DNA fragmentation assay
For the DNA fragmentation assay, low-molecular-weight DNA was isolated [13]. Briefly, 3 × 106 cells were seeded in each 100-mm plate and treated with KP372-1 for 1 or 2 days in a serum-free condition. Both floating and attached cells were scraped and collected in medium, washed three times with phosphate-buffered saline, and resuspended in 1 ml of lysis buffer (20 mM Tris HCl [pH 8], 10 mM ethylenediaminetetraacetic acid [pH 8], and 0.5% Triton X-100). After incubation on ice for 30 min, the lysates were spun at 12,000 rpm in a microcentrifuge for 10 min. Low-molecular-weight DNA in the supernatant was extracted with equal volumes of phenol and chloroform for 1 h at 4°C. Ammonium acetate (2 M) was added to the aqueous phase, and DNA was precipitated with two volumes of ethanol at −20°C overnight. DNA was treated with RNase A (1 mg/ml) at 37°C for 1 h, and total DNA was analyzed using 1.5% agarose gel and visualized by ethidium bromide staining of the gel.
Akt kinase assay
To test for Akt kinase activity, cells were washed twice in cold phosphate-buffered saline and lysed on ice with 500 nl of lysis buffer containing 20 mM Tris (pH 7.5), 150 mM sodium chloride, 1 mM ethylenediaminetetraacetic acid, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Equal amounts of lysates (300 ng of protein) were precleared by centrifugation and reabsorbed with protein A–protein G (1:1) agarose slurry.
Immunoprecipitation was carried out for 18 h using an immobilized anti-Akt1G1 monoclonal antibody cross-linked to agarose. Immunoprecipitates were washed three times with lysis buffer and twice with Akt kinase buffer (25 mM Tris [pH 7.5], 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, and 10 mM MgCl2). Kinase assays were performed for 30 min at 30°C under continuous agitation in kinase buffer containing 200 μM ATP and 1 ng of glycogen synthase kinase 3 (GSK-3) fusion protein as a substrate, according to the manufacturer’s instructions (Cell Signaling Technology). The incorporation of phosphate into glycogen synthase kinase 3 was assessed by Western blot analysis with an anti-phospho-specific GSK-3 α/β (Ser21/9) antibody and horseradish peroxidase–conjugated anti-rabbit antibody. To assess the level of expression of Akt, parallel total cell lysates were analyzed by Western blot analysis with a polyclonal antibody against human Akt.
Results
Akt in head and neck cancer cell lines
To show that the Akt kinase is activated in the HNSCC cell lines, we profiled the expression of pAkt, total Akt, and Akt kinase activity in a panel of head and cancer cell lines using Western blotting. As shown in Figure 2A, most of the HNSCC cell lines expressed readily detectable levels of pAkt/Ser473, pAkt/Thr308, and total Akt. The levels of pAkt were quite variable, whereas those of β-actin and total Akt were relatively consistent. Most cell lines also expressed pAkt in the absence of serum (data not shown), indicating that Akt was constitutively active in some HNSCC cells. With the exception of Tu167 and UM SCC1, all cell lines had high Akt activity in Akt immunoprecipitates (Fig. 2B).
Figure 2.
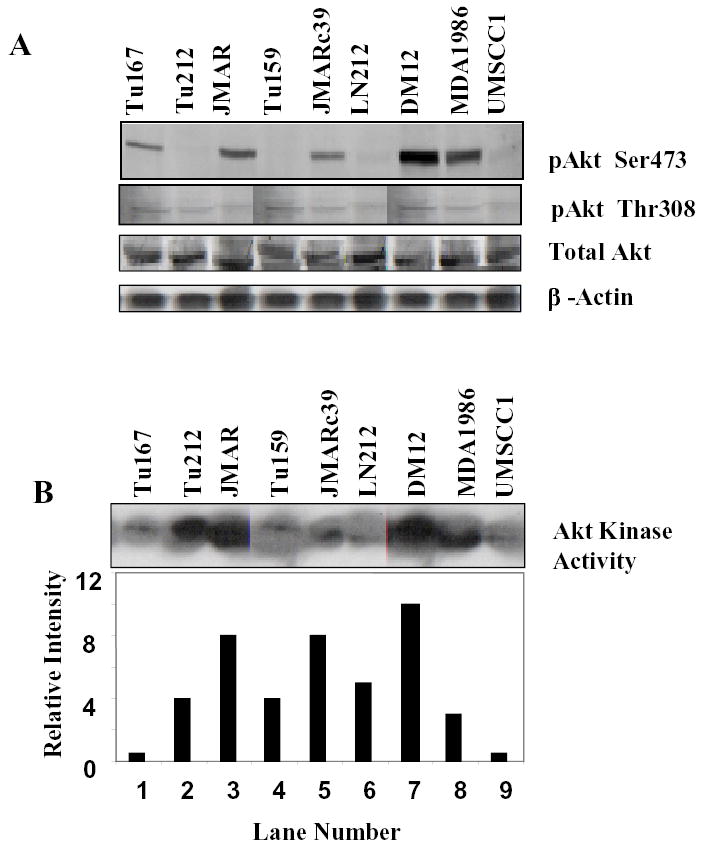
(a) pAkt/Ser473, pAkt/Thr308, and total Akt expression in different head and neck cancer cell lines by Western blotting. (b) Akt kinase activity was measured as described in Materials and Methods. The average expression of Akt kinase activity from two separate experiments was determined by densitometry and is shown in the graph below the blots.
Two cell lines were selected for further characterization: Tu167, which was derived from a floor-of-mouth squamous cell cancer, and JMAR, a subclone of Tu167 selected for resistance to anoikis, which forms more aggressive cancers than does Tu167 in an orthotopic model of tongue cancer in nude mice [12].
Akt expression in head and neck cancer tissue specimens
To determine whether the data from the HNSCC cell lines accurately reflect the situation in patients, we performed Western blotting using phosphorylation-specific antibodies against pAkt/Ser473, pAkt/Thr308, and total Akt to determine the levels of Akt and pAkt in human HNSCC specimens and the adjacent normal mucosa. As shown in Figure 3, five of eight tumors showed higher levels of Akt/Ser473 phosphorylation than did neighboring normal mucosa, and seven of eight tumors showed higher levels of Akt/Thr308 phosphorylation than did neighboring normal mucosa, suggesting an association between a high level of Akt phosphorylation and the development of head and neck cancer.
Figure 3.
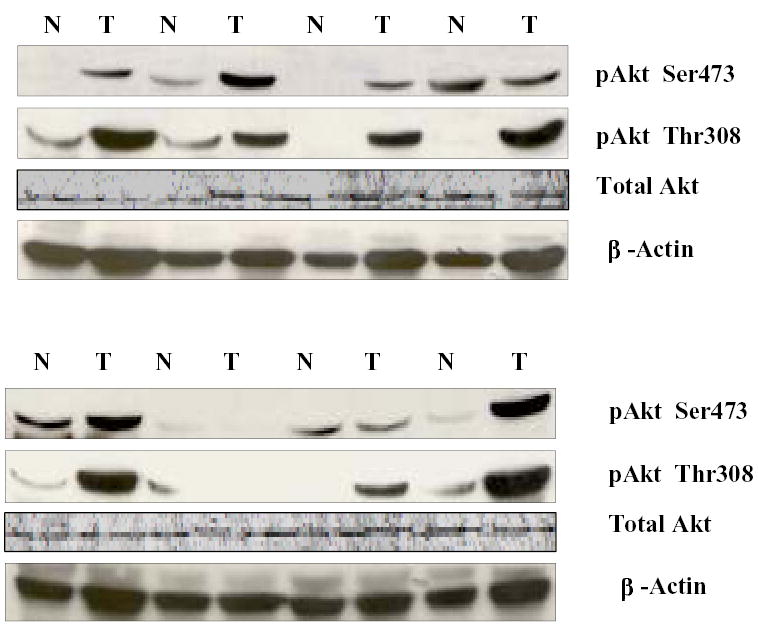
Expression of phosphorylated (p) Akt/Ser473, Akt/Thr308, total Akt, and β-actin in head and neck tumors (T) and adjacent normal mucosa (N).
KP372-1 inhibits Akt kinase activity, phosphorylation of Akt, and downstream targets of Akt in head and neck cancer cells
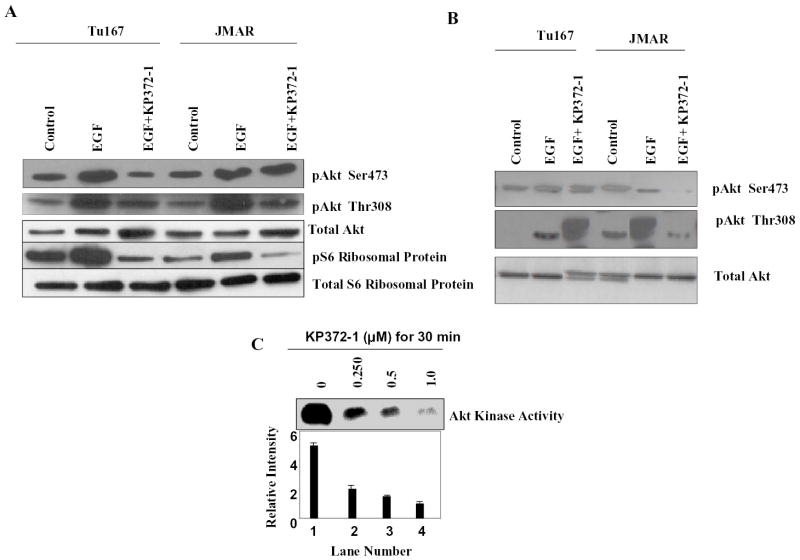
Because Akt is activated in head and neck cell lines and fresh human tumor specimens, we investigated the effects of a specific Akt kinase inhibitor, KP372-1, on Akt kinase activity, cell proliferation, apoptosis, and resistance to anoikis. We first determined the effect of KP372-1 on the Akt phosphorylation of Ser473, Thr308, and the downstream target of the Akt p-S6 ribosomal protein (Ser240/244). KP372-1 induced a small but consistent decrease in Akt phosphorylation with a concomitant marked decrease in S6 phosphorylation in both cell lines (Fig. 4A). In Fig. 4A it is shown that EGF induced the phosphorylation of Akt Ser473, Thr308 and the downstream target of the Akt p-S6 ribosomal protein (Ser240/244) but the degree of induction are different in both the cells. KP372-1 is very effective to inhibit the EGF induced phosphorylation of Aktser473 in Tu167 and AktThr308 in JMAR. KP372-1 is also very effective to inhibit the downstream target of the Akt p-S6 ribosomal protein (Ser240/244) in both the cells. The modest increase in total Akt in the KP372-1 lane was due to a failure of the total Akt antibody to bind to phosphorylated Akt and does not signify a true increase in Akt levels. Higher concentration of KP372-1 (1uM) is also very effective to inhibit the phosphorylation of Akt Ser473 and Thr308 in both the cells (Fig-4B). These results suggest that KP372-1 blocks Akt, thereby decreasing the phosphorylation of the S6 ribosomal protein. The mechanisms responsible for the decrease in Akt phosphorylation are unknown; this decrease may be due to changes in the conformation of Akt because KP372-1 has not demonstrated activity against other kinases, including PDK1 and ILK (QLT; unpublished), which can act as a PDK2.
Figure 4.
KP372-1 inhibits Akt phosphorylation, some downstream signaling molecules, and Akt kinase activity. (a) Tu167 and JMAR cells were treated with 20 ng/ml epidermal growth factor (EGF) or with EGF plus 125 nM KP372-1 for 30 min. Equal amounts of cell lysate proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with different antibodies as indicated. (b) Tu167 and JMAR cells were treated with 20 ng/ml epidermal growth factor (EGF) or with EGF plus 1uM KP372-1 for 30 min. Equal amounts of cell lysate proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with different antibodies as indicated. (c) KP372-1 inhibits Akt kinase activity. JMAR cells were treated with the different concentrations of KP372-1 for 30 min, and cell lysates were analyzed for Akt kinase activity and total GSK-β. The mean (±SD) expression of Akt kinase activity from three separate experiments was determined by densitometry and is shown in the graph below the blots.
Next, we determined the effects of KP372-1 on Akt kinase activity in JMAR cancer cells (Fig. 4C). We treated the JMAR cells with different concentrations of KP372-1 for 30 min and evaluated Akt kinase activity in an in vitro kinase assay. KP372-1 significantly blocked Akt kinase activity in a dose-dependent fashion, with an IC50 (concentration at which 50% inhibition is achieved) of 250 nM, which was compatible with the dose required to inhibit PI-3K/Akt signaling in intact cells.
KP372-1 inhibits proliferation and induces apoptosis of head and neck cancer cells
The effect of KP372-1 on the growth of both JMARc42 and Tu167c2 cells was evaluated using the MTT assay. The proliferation of both cell lines was inhibited by KP372-1, with an IC50 of 200 nM for JMARc42 cells and 100 nM for Tu167c2 cells (Fig. 5). Because changes in cell proliferation can be caused by alterations in either cell cycle progression or apoptosis, we examined whether the effects of KP372-1 on cell growth were due partly to apoptosis induction. We treated the Tu167 or JMAR cells with increasing concentrations of KP372-1 and determined the extent of apoptosis on the basis of DNA fragmentation, as measured by ethidium bromide staining (Fig. 6A), and the accumulation of a sub-G0/G1 cell population, as shown by flow cytometry (Fig. 6B). Tu167 is very sensitive to KP372-1, as shown by the fact that approximately 90% of cells undergo apoptosis after incubation for 24 h with 125 nM KP372-1 (Figs. 6A and 6B). The more aggressive cell line, JMAR, was less sensitive to KP372-1. The effect of KP372-1 on the status of Bax and poly (ADP-ribose) polymerase, known apoptotic markers, was also examined (Fig. 6C). To determine the duration of exposure to KP372-1 required to commit cells to apoptosis, Tu167 and JMAR cells were incubated with KP372-1 at the IC50 for 6 h in serum-free medium, washed with phosphate-buffered saline, and then grown in a medium containing 10% fetal calf serum without KP372-1 for another 24 or 48 h before being assayed for apoptosis. Apoptosis was not induced under these conditions (data not shown). Thus, we concluded that KP372-1 must be continuously present to induce apoptosis.
Figure 5.
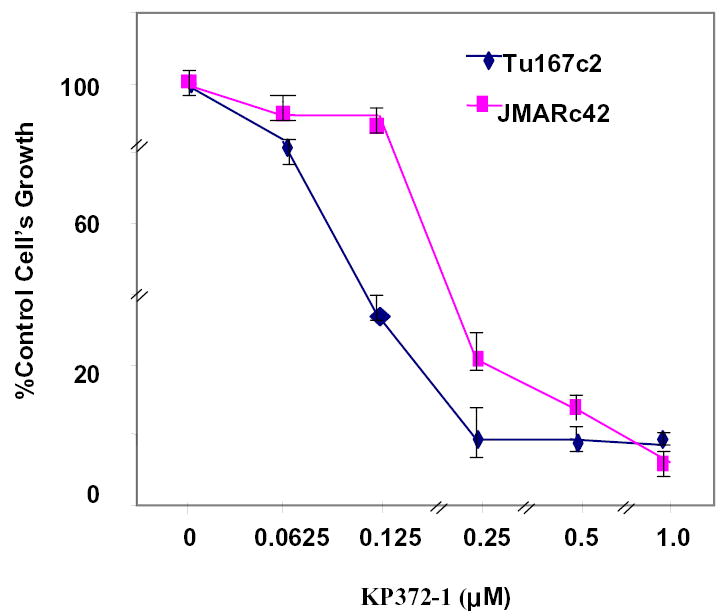
Effects of KP372-1 on cell growth. Tu167c2 and JMARc42 cells were plated in a 96-well plate and treated with different concentrations of KP372-1 for 48 h. Cell growth was measured by an MTT assay as described in Materials and Methods. Results shown are representative of three separate experiments.
Figure 6.
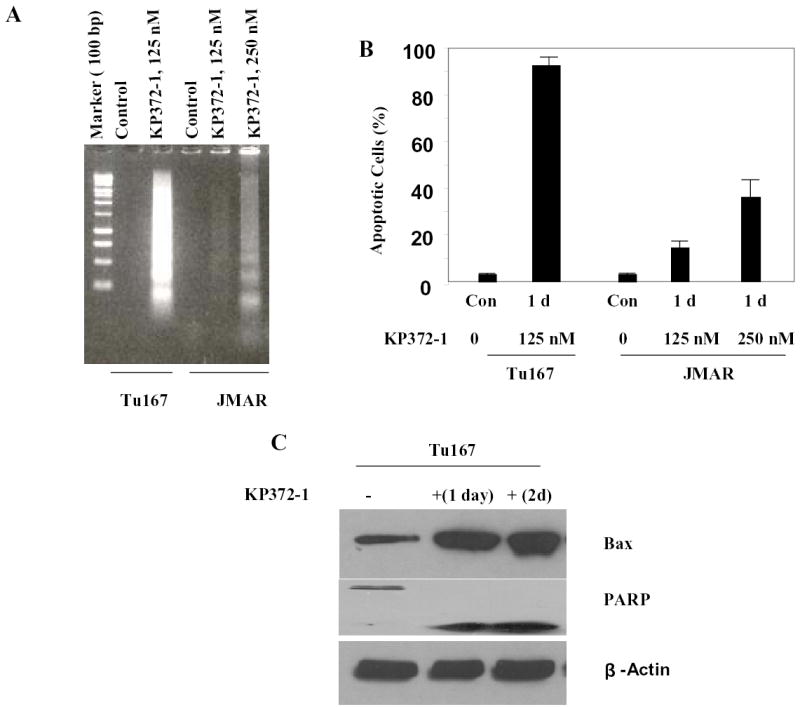
KP372-1 induces apoptosis in head and neck cancer cells. (a) Cells were cultured with the specified concentrations of KP372-1 for 1 day (1 d), and DNA fragmentation was measured by ethidium bromide staining. (b) Cells were treated with the specified concentrations of KP372-1 for 1 day, and the percentage of apoptotic cells was measured by flow cytometry. (c) Tu167 cells were treated with 125 nM KP372-1 for the indicated periods, and cell extracts were immunoblotted with the indicated antibodies. Results shown are representative of three separate experiments with similar results. Con, control; PARP, poly (ADP-ribose) polymerase.
KP372-1 induces anoikis in head and neck cancer cells
Because Akt signaling has previously been shown to be important in mediating survival signals under anchorage-independent conditions in some tumor cell lines [14], we evaluated the effect of Akt inhibition on the induction of apoptosis under both attached and detached conditions in the anoikis-sensitive Tu167 cells and the anoikis-resistant JMAR cells. As shown in Figure 7, KP372-1 induced anoikis in the JMARc42 cells at concentrations as low as 125 nM, with 70% of cells showing apoptosis after 24 h in suspension culture; less than 10% of cells treated similarly under attached conditions showed apoptosis. Taken together, these findings showed that the inhibition of Akt blocked growth and induced apoptosis and anoikis in head and neck cancer cells.
Figure 7.
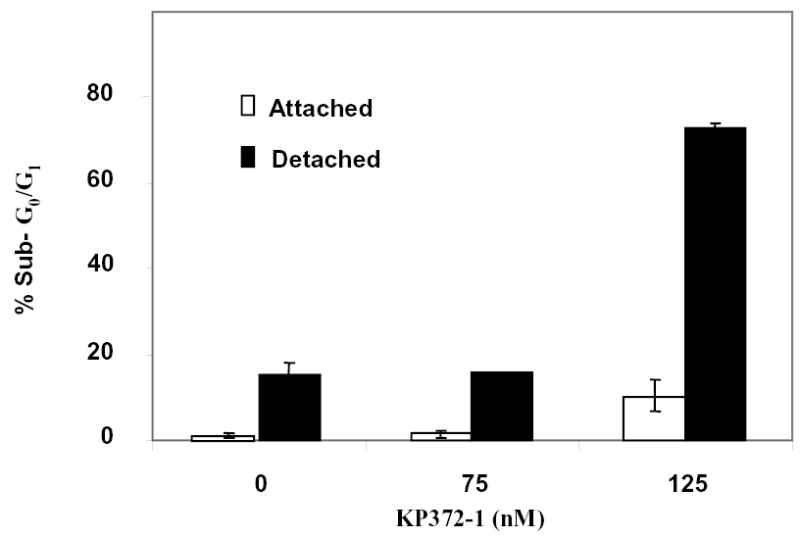
Effect of KP372-1 on anoikis in JMARc42 cells. Detached cells were suspended in a 15-ml tube with or without KP372-1 and rotated in the incubator for 24 h, as described in Materials and Methods. Sub-G0/G1 cells were assessed by flow cytometry. Results shown are representative of four separate experiments with similar results.
Discussion
Our study showed that most head and neck cancer cell lines express detectable levels of Akt/Ser473, Akt/Thr308, total Akt, and Akt kinase activity. Similarly, in comparisons of HNSCC tumors and matching normal mucosa, several HNSCC tumors showed a substantial increase in Akt/Ser473 activity, Akt/Thr308 phosphorylation, and Akt kinase activity, suggesting a close association between a high level of Akt phosphorylation and cancer of the head and neck. Compatible with a role for the PI-3K/Akt pathway in the pathophysiology of head and neck cancer, the blockade of Akt signaling by the selective inhibitor KP372-1 induced apoptosis and anoikis and inhibited cell proliferation in human oral cancer cells growing in culture. In vitro, KP372-1 inhibited the phosphorylation and kinase activity of Akt and the phosphorylation of downstream substrates. This inhibition was associated with the induction of Bax expression and cleavage of poly (ADP-ribose) polymerase, which potentially contribute to the apoptosis-and anoikis-inducing effects of KP372-1. KP372-1 also inhibits other kinases, such as CDK1, CK2, CSK, DNAPK, ERK1, GSK3b, LCK, MEK1, PIM, PKA, PKC, and S6K, albeit at relatively high concentrations (unpublished work from QLT).
Activation of Akt appears to be a key step in the development of SCC [15] because Akt activation has been linked to the multi-stage progression of HNSCC [16]. This increase in Akt activity in HNSCC was also found in a large fraction (70%) of HNSCC-derived cell lines, irrespective of whether the EGF receptors were activated in these cells [17]. In a recent study, a significant association was found between pAkt activation and decreased local tumor control in a series of 38 patients [18].
Amornphimoltham et al. [16] recently showed that UCN-01, which inhibits the Akt pathway by inhibiting PDK1, displayed potent antiproliferative properties in HNSCC cell lines, leading to apoptosis, and that, in HNSCC tumor xenograft models, treatment with UCN-01 for 5 consecutive days led to sustained inhibition of tumor growth, even after only one cycle of UCN-01 treatment. These effects were associated with a high incidence of apoptosis, a decrease in cyclin D3 levels, and an increase in p27KIP1 expression in treated tissues. Thus, these observations, taken together with the frequent occurrence of Akt activation in HNSCCs and their cell lines, indicate that the Akt pathway could be a useful target for therapy.
That Akt could be a critical target for cancer intervention in HNSCC is further supported by the work of Yang et al. [19], who found that Akt/protein kinase B signaling inhibitor 2 suppressed the kinase activity and phosphorylation level of Akt. Furthermore, the inhibition of Akt kinase activity suppressed cell growth and induced apoptosis in human cancer cells that harbor constitutively activated Akt owing to the overexpression of Akt or other genetic alterations such as a phosphatase and tensin homolog mutation. Akt/protein kinase B signaling inhibitor 2 is highly selective for Akt and does not inhibit the activation of PI-3K, phosphoinositide-dependent kinase-1, protein kinase C, serum-glucocorticoid–inducible kinase, protein kinase A, signal transducers and activators of transcription 3, extracellular signal–regulated kinase-1/2, or c-Jun NH2-terminal kinase. Furthermore, in nude mice, Akt/protein kinase B signaling inhibitor 2 potently inhibited the growth of human cancer cells in which Akt was aberrantly expressed or activated but not that of cancer cells in which Akt expression or activation was normal [19]. These findings strongly support the development of anticancer agents that target Akt; such small-molecule Akt inhibitors could have wide applicability as anticancer drugs.
Further studies have shown that inhibition of the PI-3K/Akt pathway by biochemical or genetic means increases the in vitro and in vivo efficacy of chemotherapy, radiotherapy, or both [20,21]. Several standard chemotherapeutic and chemopreventive agents inhibit the PI-3K/Akt pathway when they are administered in vitro, and in some cases, the inhibition of Akt is directly responsible for these agents’ cytotoxicity [22].
Despite the widely acknowledged potential of Akt inhibitors as anticancer therapy, few have made it to clinical trials. KP372-1 compares favorably in vitro with other inhibitors of the PI-3K/Akt pathway, such as Wortmannin or LY294002, and with nonspecific small-molecule kinase inhibitors engineered for greater specificity toward Akt [23]. Wortmannin and LY294002 may have limited clinical utility owing to their lack of specificity, associated adverse effects, poor pharmacology, and poor solubility [23]. Wortmannin also inhibits myosin light-chain kinase; phospholipases C, D, and A2; and DNA-dependent protein kinase [23], and LY294002 inhibits the aryl hydrocarbon receptor, a ligand-activated transcription factor [24]. In vivo use of LY294002 in mice has been associated with many adverse effects, including death [25]. Furthermore, in mice, Wortmannin induces liver and bone marrow toxicity, and LY294002 can induce dermatitis and inhibit growth. Therefore, although Wortmannin and LY294002 inhibit the PI-3K/Akt pathway, their drawbacks raise doubts about their suitability as leading candidates for additional drug development.
Whether the specific inhibition of Akt will be clinically valuable will also depend on the spectrum of toxic effects associated with its use in animal models, because Akt may be critically important in signaling transduction for other physiologic processes such as signaling through the insulin receptor [26]. Therefore, rigorous testing in preclinical models will be needed before clinical trials of Akt and Akt-targeted compounds can be undertaken.
In summary, HNSCC cell lines and human tumor specimens showed high levels of Akt phosphorylation of Ser473 and Thr308 and high levels of Akt activity, suggesting that the Akt signaling pathway plays a role in HNSCC progression. In addition, specific inhibition of the Akt kinase by KP372-1 decreased the cell proliferation of HNSCC cells and induced apoptosis and anoikis in them in vitro. Taken together, our results indicate that the PI-3K/Akt pathway is an important potential therapeutic target in HNSCC and that further preclinical evaluation of KP372-1 is warranted.
Footnotes
Grant support: Supported in part by National Institutes of Health grants Head & Neck SPORE 1 P50 CA097007 and RO1-DE14613 (J.N. Myers) and P50 CA83639 (G.B. Mills).
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics. 2003;53(1):5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Myers EN, Suen JY. Cancer of the head and neck, 3rd ed. Philadelphia: Saunders, 1996.
- 3.Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92(12):3030–6. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Mills GB, Lu Y, Fang X, Wang H, Eder A, Mao M, et al. The role of genetic abnormalities of PTEN and the phosphatidylinositol 3-kinase pathway in breast and ovarian tumorigenesis, prognosis, and therapy. Semin Oncol. 2001;28(5 Suppl 16):125–41. doi: 10.1016/s0093-7754(01)90290-8. [DOI] [PubMed] [Google Scholar]
- 5.Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61(15):5911–8. [PubMed] [Google Scholar]
- 6.Worsham MJ, Pals G, Schouten JP, Van Spaendonk RM, Concus A, Carey TE, et al. Delineating genetic pathways of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129(7):702–8. doi: 10.1001/archotol.129.7.702. [DOI] [PubMed] [Google Scholar]
- 7.Woenckhaus J, Steger K, Werner E, Fenic I, Gamerdinger U, Dreyer T, et al. Genomic gain of PIK3CA and increased expression of p110alpha are associated with progression of dysplasia into invasive squamous cell carcinoma. J Pathol. 2002;198(3):335–42. doi: 10.1002/path.1207. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama H, Ikebe T, Beppu M, Shirasuna K. High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92(12):3037–44. doi: 10.1002/1097-0142(20011215)92:12<3037::aid-cncr10171>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430(7003):1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 10.Zhan M, Zhao H, et al. Signalling mechanisms of anoikis. Histol Histopathol. 2004;19(3):973–83. doi: 10.14670/HH-19.973. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Price JE, Fan D, Zhang RD, Bucana CD, Fidler IJ. Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. J Natl Cancer Inst. 1989;81(18):1406–12. doi: 10.1093/jnci/81.18.1406. [DOI] [PubMed] [Google Scholar]
- 12.Swan EA, Jasser SA, Holsinger FC, Doan D, Bucana C, Myers JN. Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol. 2003;39(7):648–55. doi: 10.1016/s1368-8375(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 13.Mandal M, Maggirwar SB, Sharma N, Kaufmann SH, Sun SC, Kumar R. Bcl-2 prevents CD95 (Fas/APO-1)-induced degradation of lamin B and poly(ADP-ribose) polymerase and restores the NF-kappaB signaling pathway. J Biol Chem. 1996;271(48):30354–9. doi: 10.1074/jbc.271.48.30354. [DOI] [PubMed] [Google Scholar]
- 14.Davies MA, Lu Y, Sano T, Fang X, Tang P, LaPushin R, et al. Adenoviral transgene expression of MMAC/PTEN in human glioma cells inhibits Akt activation and induces anoikis. Cancer Res. 1998;58(23):5285–90. [PubMed] [Google Scholar]
- 15.Segrelles C, Ruiz S, Perez P, Murga C, Santos M, Budunova IV, et al. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002;21(1):53–64. doi: 10.1038/sj.onc.1205032. [DOI] [PubMed] [Google Scholar]
- 16.Amornphimoltham P, Sriuranpong V, Patel V, Benavides F, Conti CJ, Sauk J, et al. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10(12 Pt 1):4029–37. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- 17.Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003;63(11):2948–56. [PubMed] [Google Scholar]
- 18.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8(3):885–92. [PubMed] [Google Scholar]
- 19.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64(13):4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 20.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1(12):989–97. [PubMed] [Google Scholar]
- 21.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61(10):3986–97. [PubMed] [Google Scholar]
- 22.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62(4):1087–92. [PubMed] [Google Scholar]
- 23.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5(6):234–48. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 24.Guo M, Joiakim A, Reiners JJ., Jr Suppression of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated aryl hydrocarbon receptor transformation and CYP1A1 induction by the phosphatidylinositol 3-kinase inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1- benzopyran-4-one (LY294002) Biochem Pharmacol. 2000;60(5):635–42. doi: 10.1016/s0006-2952(00)00379-8. [DOI] [PubMed] [Google Scholar]
- 25.Hu L, Zaloudek C, Mills GB, Gray J, Jaffe RB. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002) Clin Cancer Res. 2000;6(3):880–6. [PubMed] [Google Scholar]
- 26.Bevan P. Insulin signalling. J Cell Sci. 2001;114(Pt 8):1429–30. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]