Abstract
Purpose
Fixation at the osteotomy site for mandibular distraction osteogenesis (DO) is probably not rigid, especially during mastication. Micromotion may affect the course of DO. This study aimed to measure the mobility of the fresh distractor-fixed osteotomy site in response to mastication and masticatory muscle stimulation.
Materials and methods
28 domestic pigs, 6–8 weeks old, underwent osteotomy of the right mandible and placement of a distractor appliance. Immediately after surgery, displacement at three different locations (superior-lateral, inferior-lateral and inferior-medial) of the osteotomy site was assessed using ultrasound piezoelectric crystals and/or differential variable reluctance transducers (DVRTs). The amount of lengthening or shortening at each location was measured during mastication and muscle stimulation. Displacement was also measured for bilateral osteotomy during muscle stimulation from a subgroup of 12 pigs.
Results
The osteotomy site demonstrated significant mobility during power strokes of mastication with an average magnitude of 0.3–0.4 mm. Distinct patterns of displacement were associated with different locations and the patterns varied between chewing sides. The most common pattern was lengthening at the superior-lateral and shortening at both inferior sites. Similar amounts of displacement were observed during the stimulation of jaw-closers (masseter and medial pterygoid), but the patterns produced by these muscles did not completely explain the masticatory pattern. Opening the osteotomy to 1.5 mm did not alter the displacements observed during muscle stimulation. Bilateral osteotomy tended to decrease displacement.
Conclusions
The study demonstrates that during mastication and masticatory muscle stimulation, an acute mandibular osteotomy site is mobile despite fixation by a distractor appliance.
Keywords: Distraction osteogenesis, mandible, osteotomy, micromotion, mastication, pig
INTRODUCTION
Distraction osteogenesis (DO) of the mandible has become increasingly popular for treating a myriad of craniofacial malformations previously requiring major osteotomies and bone grafts1–5. DO is a mechanical process. Tensile force produced by the distractor is considered the main stimulus for osteogenesis at the osteotomy6, which otherwise would only undergo a fracture healing process. This tensile force is assumed to be the only important load on the osteotomy site, which is bridged and fixed by the distractor appliance. Physiologically, however, the mandible is heavily loaded by forces from occlusion and masticatory muscle contraction7–10. Our previous work on the zygomatic suture found that even rigid fixation allowed considerable movement between two opposing bones, suggesting that the stiffness of the distractor appliance might not completely stabilize the osteotomy. From a mechanical view, at least two factors make likely the interfragmentary movement of a mandibular DO site. First, the distractor appliance is usually placed on the lateral surface, far from the midline center of gravity11. Second, the distractor only takes up a small portion of the wide mandibular surface. These factors work against the ability of the appliance to stabilize the mandible in space.
Instability of the osteotomy site is not necessarily a liability. Molina and Ortiz Monasterio4 have advocated the use of a relatively flexible apparatus that allows curvature during the distraction procedure. For fracture healing, small amounts of interfragmentary strain may either be beneficial (axial movement)12 or harmful (shear movement)13–14. For DO, however, little is known even about the mechanics of the major tensile action15–16 and nothing has been published about the intermittent movements that must accompany contraction of the masticatory muscles. Instability of the osteotomy could either enhance or detract from the distraction, and varying strains at different parts of the osteotomy could account for the uneven regenerates reported in several mandibular DO studies17–19.
As an initial step, the current study was undertaken to investigate the mobility of an acute (at the time of osteotomy) DO site at the mandibular angle. At this phase, the DO site is in fact a fresh fracture. The stability of different fixators and methods has been extensively investigated for mandibular angle fractures20–23. These in vitro studies suggest that even the strongest fixation still permits interfragmentary micromotion. However, these studies, based only on measurement from cadavers or fabricated molds with simulation of masticatory mechanics, provide rather limited information about how the osteotomy is deformed under physiological conditions. Moreover, the distractor differs from most fracture fixators in structure and means of placement, raising the possibility of different mechanics.
Following a number of other recent mandibular DO investigations24–25, a pig model was used in this study. The similarity of mandibular anatomy and masticatory mechanics between the pig and the human26–28 makes this species particularly suitable for investigation. The mobility at different locations of the DO site was measured in vivo during mastication and stimulation of masticatory muscles. The results demonstrate that the mandibular DO site is distorted in predictable patterns by mastication and masticatory muscle contraction.
MATERIALS AND METHODS
Animals
A total of 28 domestic pigs were used in two groups (Group 1, #1–16; Group 2, #17–28). All pigs were about 6–8 weeks old, an age roughly equivalent to the primary dentition stage of a 3–6 year-old child. After about 1 week acclimation to the laboratory environment, animals were verified to have normal chewing patterns by electromyographic (EMG) recording of mastication using techniques described previously29. Several days later, a terminal experiment was performed, during which time pigs received surgical osteotomy and implantation of a distractor appliance and measuring devices. Recordings were made during chewing and masticatory muscle stimulation. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Terminal experimental procedures
Group 1
Animals were anesthetized by mask using isoflurane and nitrous oxide. An incision was made along the lower border of the right mandible. After reflecting supra-periosteally the anterior 1/3 of the masseter attachment, the junction between mandibular body and ramus was exposed, where an oblique corticotomy, about 60° to the occlusal plane, was made using a Stryker saw (Kalamazoo, MI) (Fig. 1). A Synthes® (Monument, CO) or a KLS-Martin® (Jacksonville, FL) distractor appliance was implanted perpendicular to the corticotomy as presented in more detail elsewhere30. The osteotomy was then completed and Gelfoam (Pharmacia & Upjohn, Kalamazoo, MI) was placed for hemostasis. Strain gauges were placed on the mandibular body and condylar neck as described in Part II of this study31.
Figure 1.
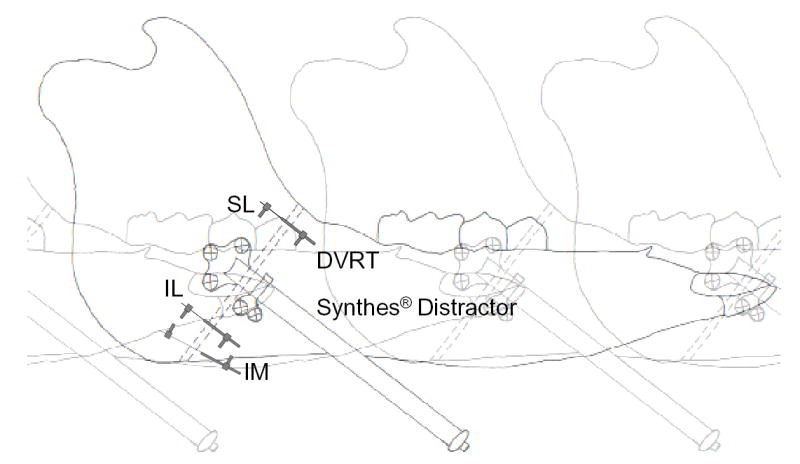
Schematic drawing of the pig right mandible showing the osteotomy (broken lines) fixed by a Synthes® appliance in an anterior exit configuration and DVRT (Group 2) placement at three different locations. The osteotomy site is drawn as a relatively wide gap only for clarity. SL, superior-lateral; IL, inferior-lateral; IM, inferior-medial. For Group 1 pigs, sonometric crystal pairs instead of DVRTs were placed at the former two locations.
Devices to measure the deformation of the distraction gap were then implanted. These consisted of six piezoelectric crystals (Sonometrics, London, Ont., Canada) on the lateral side and a differential variable reluctance transducer (DVRT; Microstrain, Williston, VT) on the less accessible medial side. The crystals, 2 mm in diameter, were placed in pairs on opposite sides of the osteotomy gap. One pair was glued to the bone screws of the distractor plates using tissue adhesive, while the other two pairs, fitted with plastic pegs, were placed on the lateral bone surfaces superior (SL location) or inferior (IL location) to the distractor appliance through insertion of their pegs into drilled holes. These crystals were used for digital sonomicrometry. Real-time displacement between each pair of crystals was recorded with a theoretical resolution of 0.02 mm. To facilitate signal transmission, ultrasound conductivity gel (Liqui-Cor, Spacelabs Burdick, Inc., Deerfield, WI) was injected to cover the crystals before closing the incision. The DVRT has two coils, which were secured to the inferior-medial (IM location) bone surfaces on opposite sides of the osteotomy through insertion of their attached screws into drilled holes. Displacement between bones resulted in sliding of one coil on the other with a theoretical sensitivity of 0.001 mm.
The incision was then closed. In a subsample (6 pigs) of these animals, an additional pair of crystals was implanted on the intact left side after partial reflection of the masseter. This pair, placed approximately in the IL location, served to evaluate experimental displacement in the absence of osteotomy. Then, fine-wire EMG electrodes were inserted into the masseter and temporalis on both sides. Ketorolac tromethamine (Abbott Labs, N. Chicago, IL), 1 mg/Kg IM, and Buprenorphine hydrochloride (Reckitt and Colman, Richmond, VA), 0.005 mg/Kg IM were given for general analgesia. 1% lidocaine was applied topically to the incisions. The pigs were allowed to awaken and eat their regular diet of pelleted chow, during which deformation and EMG were recorded. The hard diet was preferred to softened chow by the pigs and caused clearer movements and transducer signals. Via a transceiver, crystal signals were collected to a computer running the Sonometrics software. DVRT signals, together with EMG and strain signals, were collected by another computer running Acqknowledge III software (Biopac Systems, Santa Barbara, CA).
After about 10 minutes of chewing, the pigs were re-anesthetized. Stimulating electrodes were inserted into bilateral masseter, medial pterygoid, digastric and lateral pterygoid muscles. For each muscle, two electrodes were placed approximately at the maximum diagonal perpendicular to the long axis of the muscle. The anesthetized pig was placed prone on the table. After determining the voltage level for supramaximal contraction (usually 20–50 V depending on muscle), each muscle was tetanized unilaterally and bilaterally with 0.5 Hz trains of 600 ms, 60 pps, 5 ms pulses (Grass Model S38, Quincy, MA) while recordings of osteotomy deformation were collected. Then the distractor was activated 4 turns, which was expected to open the osteotomy 2 mm, and the same muscle stimulations were repeated. The rationale for this procedure was to ascertain whether direct contact between the fragments (as would occur in the closed but not the activated condition) was a major determinant of interfragmentary mechanics.
Group 2
Group 2 pigs received the same osteotomy of the right mandible. Each was fitted with a Synthes® distractor placed in an anterior-exit configuration. Strain gauges were implanted as described in Part II31. Then, DVRTs were implanted across the osteotomy at all three bone locations (SL, IL and IM, Fig. 1), except for the first three pigs, which used ultrasound crystals at the SL and IL locations. After recordings like those for Group 1 pigs were made during chewing and muscle stimulation, a second osteotomy was made on the left mandible and three pairs of ultrasound crystals were placed at the same three locations across the osteotomy. Muscle stimulations were then repeated and recordings were made, first with the distractors closed and then with the distractors activated 4 turns.
Following collection of these data, pigs from both groups were sacrificed with an intra-cardiac injection of a lethal dose of pentobarbital.
Data analysis
For each location, displacement during the chewing cycle was obtained by subtracting the average value during the opening phase, which is relatively stable with time, from the peak value during the power stroke. For each pig, at least 10 chewing cycles were measured and the displacements were averaged. EMG recordings were used to determine chewing side. The displacement during muscle stimulation was obtained by subtracting the baseline value from the muscle contraction value, and measurements of three (usually) tetani were averaged. The displacements recorded by ultrasound crystals were directly calculated, while those recorded by DVRTs were converted from voltages using previously established calibrations.
Differences in displacement magnitude were compared using Student t-tests or one-way ANOVAs, while the polarity of displacement was evaluated by binomial tests.
RESULTS
Performance of measuring devices
The ultrasound crystals performed poorly during mastication, yielding very noisy recordings with many artifacts, partly due to interference from air brought into the incision by rapid jaw movement. After discarding artifactual recordings, no animal in Group 1 had complete mastication data for all locations, and no usable data were obtained from the first three pigs in Group 2. Crystal performance was better for muscle stimulation, during which it was possible to control artifacts by injecting more conductivity gel and dispelling air from the incision.
DVRTs were more stable during mastication and most pigs in Group 2 had complete recordings. Due to the limited number of DVRT channels, the left osteotomy was still measured using ultrasound crystals. However, these recordings only involved muscle stimulation, for which both DVRTs and crystals performed well.
Displacement during mastication
Pre-operative electromyography showed that all pigs had a normal chewing pattern, alternating sides from one chewing cycle to the next. As discussed more fully in Part II31, postoperatively pigs favored the left side.
During mastication, crystals on the left intact mandible of 4 pigs functioned properly and returned an average displacement of 0.05±0.04 mm. This 0.05 mm represents an estimate of experimental error, which was probably caused by the movement of overlying tissues and by minor bone deformation. On the right side, displacement of the distractor was not significantly different from control error, as presented previously30. However, significant displacements were seen at all three bone locations. Displacement mainly occurred during the jaw-closing phase especially in relation to the power stroke. Minimal displacement sometimes accompanied the jaw-opening phase. Only the closing displacement was analyzed.
The results from Group 1 pigs are presented in Table 1. Because the EMG was not synchronized with the ultrasound signals, chewing side could not be determined. All available measurements from the inferior two locations demonstrated compression while those from the superior location varied among subjects. The average absolute mobility (regardless of displacement direction) was SL 0.55±0.46 mm, IL 0.27±0.14 mm and IM 0.28±0.17 mm, all significantly higher than experimental error (2-sample t-tests, p<0.05).
Table 1.
Distraction site displacement (mean values) during mastication (Group 1)
| Displacement (mm)
|
||||
|---|---|---|---|---|
| Pig No. | Weight (Kg) | SL | IL | IM |
| 1 | 9.1 | −0.77 | ND | ND |
| 2 | 14.5 | ND | ND | ND |
| 3 | 16.5 | 0.20 | ND | −0.11 |
| 4 | 14.5 | ND | −0.30 | ND |
| 5 | 8.2 | 0.22 | −0.28 | −0.38 |
| 6 | 6.4 | ND | ND | −0.15 |
| 7 | 7.3 | −0.72 | 0.28 | ND |
| 8 | 13.6 | ND | ND | −0.60 |
| 9 | 12.5 | ND | ND | ND |
| 10 | 13.6 | ND | ND | ND |
| 11 | 9.1 | 0.14 | −0.03 | ND |
| 12 | 11.3 | −0.35 | −0.13 | −0.21 |
| 13 | 13.2 | ND | ND | −0.15 |
| 14 | 15.9 | ND | −0.42 | −0.21 |
| 15 | 14.5 | 1.43 | −0.42 | −0.50 |
| 16 | 10.0 | ND | ND | −0.24 |
Positive displacements: lengthening; negative displacements: shortening. ND, no data.
Displacement from Group 2 pigs is presented in Table 2. Synchronization of DVRT with EMG signals enabled chewing sides to be distinguished. A consistent displacement pattern was observed for left chews. The peak displacement at the SL location was lengthening, and the two inferior locations shortened (Fig. 2A). For right chews, only limited numbers of recordings were obtained and for these both the SL and IM locations usually showed shortening, while the IL displacement was erratic (Fig. 2B, Table 2). To estimate the total mobility at each location, an absolute value with chewing sides combined was calculated (Table 2), resulting in averages of SL 0.35±0.19 mm, IL 0.27±0.17 mm and IM 0.30±0.11 mm, all significantly higher than experimental error (2-sample t-tests, p<0.01). These peak displacements were not the only ones seen during mastication. Some locations showed gradual displacement during the opening phase (IM, Fig. 2A). Commonly, a set of lesser displacements immediately preceded the peak values. These frequently resembled chewing on the opposite site (for example, for the left chewing in Fig. 2A, the peak values are positive for SL and negative for IL, but just before most of these peaks are smaller displacements of opposite polarity).
Table 2.
Distraction site displacement (mean value) during mastication (Group 2)
| Left Chew Displacement (mm)
|
Right chew Displacement (mm)
|
Total Mobility‡ (mm)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig No. | Pig Weight (Kg) | Chewing side (L/R) | SL | IL | IM | SL | IL | IM | SL | IL | IM |
| 17 | 16.0 | 30/26 | ND | ND | −0.37 | ND | ND | −0.18 | ND | ND | 0.37 |
| 18 | 16.0 | 25/0† | ND | ND | −0.44 | ND | ND | ND | ND | ND | 0.44 |
| 19 | 12.2 | 20/4 | 0.21 | −0.07 | −0.27 | −0.36 | 0.11 | 0.10 | 0.57 | 0.18 | 0.37 |
| 20 | 15.6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 21 | 6.8 | 30/5 | 0.33 | −0.14 | −0.14 | −0.33 | 0.09 | −0.12 | 0.66 | 0.23 | 0.14 |
| 22 | 5.4 | 22/0 | 0.19 | −0.19 | −0.14 | ND | ND | ND | 0.19 | 0.19 | 0.14 |
| 23 | 4.1 | 37/7 | 0.36 | −0.11 | −0.30 | 0.35 | −0.08 | −0.18 | 0.36 | 0.11 | 0.30 |
| 24 | 7.2 | 14/0† | 0.09 | −0.09 | −0.19 | ND | ND | ND | 0.09 | 0.09 | 0.19 |
| 25 | 15.8 | 21/0 | 0.19 | −0.52 | −0.42 | ND | ND | ND | 0.19 | 0.52 | 0.42 |
| 26 | 12.7 | 26/8 | 0.28 | −0.01 | ND | −0.15 | −0.57 | ND | 0.43 | 0.57 | ND |
| 27 | 22.6 | 21/0 | 0.25 | −0.26 | −0.30 | ND | ND | ND | 0.25 | 0.26 | 0.30 |
| 28 | 21.8 | 33/9 | 0.21 | −0.30 | −0.35 | −0.17 | −0.22 | −0.26 | 0.38 | 0.30 | 0.35 |
(L/R): number of left and right cycles analyzed.
assumed all left cycles due to lack of EMG.
the larger value if left and right chews had the same polarity or the sum of absolute values if polarities were opposite. ND, no data.
Figure 2.
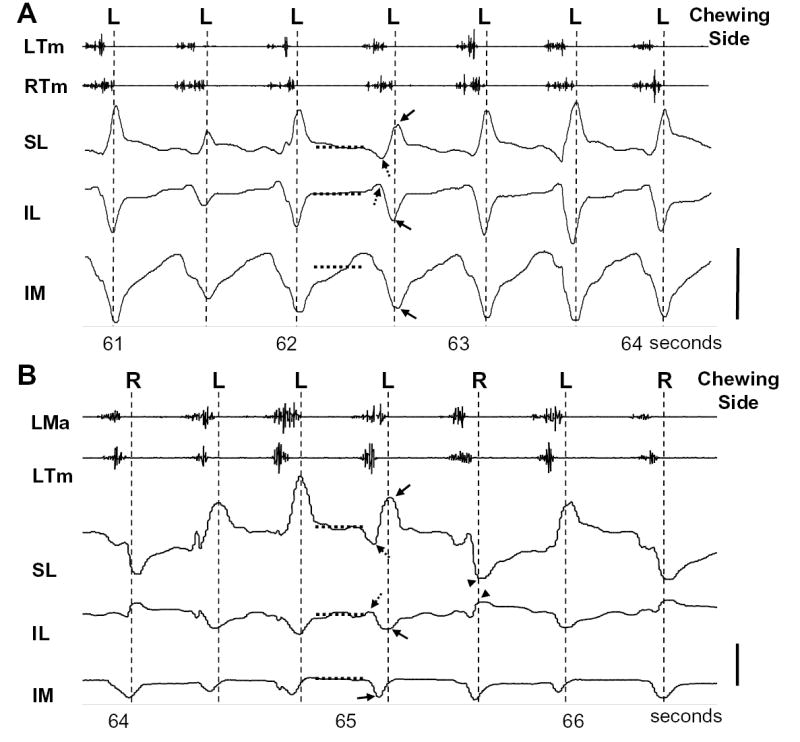
Displacement patterns of the distraction site during mastication. (A) From #27 which only had left chews, (B) from #21 which had both left and right chews. SL: superior lateral; IL, inferior lateral; IM, inferior medial. Ma, masseter; Tm, temporalis; R, right; L, left. In (A), left chews were characterized by late activity in right temporalis compared to left temporalis; in (B) left chews were characterized by late activity of left masseter compared to left temporalis. For each chewing cycle, displacement during the opening phase (no EMG) was relatively flat; approximate baselines are shown by horizontal broken lines. An upward peak in relation to the baseline is lengthening while a downward peak is shortening. Vertical broken lines mark the peak values of displacement, which occurred during the power stroke at the end of the EMG burst. Note all left chews showed peak values of SL lengthening, IL and IM shortening (arrows), while right chews reversed polarities at the SL and IL locations (arrow heads). Some chews had minor peaks (broken arrows) before the main peaks, which correspond to the closing phase of mastication when the mandible moves toward the working side. Scale bar, 0.3 mm.
Because absolute mobility was similar between the 2 groups for each location (2-sample t-tests, p>0.05), all measurements were merged. The average mobility for the combined sample was SL 0.44±0.34 mm, IL 0.27±0.15 mm and IM 0.29±0.14 mm. Although the SL location seemed to be more mobile than the two inferior locations, the difference did not reach significance (ANOVA, p=0.086). A Pearson correlation test was performed against animal weight and no significance was found for any location.
Displacement during muscle stimulation
1. Displacement after unilateral osteotomy
Displacement during the stimulation of the digastric and lateral pterygoid muscles was indistinguishable from control error. Thus these small muscles do not significantly displace the osteotomy. Because distractor types and configurations did not affect displacement30 and because displacement and animal weight were not correlated, measurements from both groups were combined for analysis of the masseter and medial pterygoid.
Consistent polarities of displacement resulted from almost all muscle stimulations (Table 3 and Figure 3). Generally, regardless of side, stimulation of the medial pterygoid muscle caused tension at the SL location and compression at the two inferior locations, the same pattern as found during left-side chews. Displacement during masseter stimulation depended on side. Stimulation of the left (contralateral) masseter caused compression at the SL location and tension at the two inferior locations, while the right (ipsilateral) masseter caused compression at the IL location and tension at the SL and IM locations. Bilateral masseter stimulation resulted in significant polarity (lengthening) only at the IM location, while the two lateral locations were displaced randomly due to the opposite effects of left and right masseter stimulations.
Table 3.
Unilateral (right) osteotomy: displacement (mm) during muscle stimulation
| LMa Stimulation
|
RMa stimulation
|
BiMa stimulation
|
Lme stimulation
|
RMe stimulation
|
BiMe stimulation
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal No. | SL | IL | IM | SL | IL | IM | SL | IL | IM | SL | IL | IM | SL | IL | IM | SL | IL | IM |
| 1 | −0.12 | ND | ND | 0.24 | −0.10 | ND | −0.15 | ND | ND | 0.83 | −0.55 | ND | 0.11 | −0.14 | ND | 0.80 | −0.68 | ND |
| 2 | −0.20 | 0.19 | ND | 0.13 | −0.13 | ND | −0.12 | −0.04 | ND | 0.20 | −0.04 | ND | −0.33 | −0.09 | −1.06 | −0.28 | −0.02 | −0.82 |
| 3 | ND | ND | 0.52 | ND | ND | 0.15 | ND | ND | 0.54 | ND | −0.14 | ND | ND | ND | ND | 0.39 | −0.06 | 0.03 |
| 4 | −0.12 | ND | ND | ND | −0.69 | ND | ND | ND | ND | ND | −0.09 | ND | ND | −0.28 | −0.05 | 0.48 | ND | ND |
| 5 | −0.01 | 1.45 | ND | 0.53 | 0.71 | ND | 0.25 | 1.55 | ND | 0.57 | 0.91 | −0.55 | 0.03 | −0.15 | −0.30 | 0.35 | 0.14 | −0.83 |
| 6 | −0.11 | 0.08 | 0.30 | 1.17 | ND | 0.05 | 0.60 | 0.56 | 0.48 | 0.95 | ND | −0.04 | 0.30 | ND | −0.88 | 0.43 | −0.07 | −0.43 |
| 7 | −0.11 | 0.46 | 0.35 | 0.37 | −0.19 | 0.24 | −0.21 | 0.49 | 0.52 | 0.41 | −0.09 | −0.03 | 0.38 | ND | −0.49 | 0.67 | 0.45 | −0.53 |
| 8 | 0.01 | ND | 0.03 | 0.18 | −0.02 | 0.10 | 0.17 | ND | 0.15 | 0.23 | −0.01 | −0.03 | 0.14 | −0.03 | −0.7 | 0.34 | −0.08 | −0.87 |
| 9 | ND | ND | 0.48 | ND | ND | 0.21 | ND | ND | 0.42 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 10 | −0.30 | 0.20 | 0.27 | 1.08 | −0.62 | ND | 0.51 | −0.08 | 0.81 | 0.52 | −0.3 | −0.39 | 0.59 | −0.39 | −1.3 | 0.52 | −0.66 | −1.43 |
| 11 | −0.08 | 0.17 | 0.86 | 0.75 | −1.05 | 0.35 | 0.16 | 0.36 | 0.81 | 0.45 | −0.08 | −0.44 | 0.53 | −0.61 | −0.73 | 0.49 | −0.46 | −0.93 |
| 12 | −0.08 | 0.17 | 0.24 | −0.05 | 0.09 | 0.07 | −0.11 | 0.49 | 0.32 | 0.07 | −0.09 | −0.01 | 0.06 | −0.41 | −0.41 | 0.11 | −0.33 | −0.37 |
| 13 | −0.22 | 1.04 | 0.30 | 0.31 | ND | 0.43 | 0.56 | −0.66 | 1.17 | 0.36 | −0.28 | −0.12 | 0.61 | −0.22 | −1.1 | 0.43 | −0.25 | −0.67 |
| 14 | −0.50 | 0.39 | 0.44 | 0.71 | −0.21 | 0.06 | 0.95 | −0.12 | 0.14 | 0.64 | −0.21 | −0.14 | 0.16 | −0.11 | −0.77 | 0.41 | −0.22 | −0.97 |
| 15 | −0.36 | ND | 1.15 | 0.32 | ND | 0.68 | 0.42 | ND | 2.34 | 0.32 | ND | −0.10 | ND | ND | −0.51 | ND | ND | −0.67 |
| 16 | −0.19 | 0.37 | 0.59 | −0.34 | ND | −0.33 | −0.70 | −0.36 | −0.05 | 0.06 | −0.32 | −0.32 | 0.18 | −0.29 | −0.64 | 0.20 | −0.25 | −0.72 |
| 17 | −0.32 | 0.33 | 0.44 | 0.53 | −0.11 | 0.03 | −0.06 | 0.21 | 0.25 | 0.52 | 0.03 | −0.28 | −0.17 | −0.06 | −0.56 | 0.28 | 0.03 | −0.28 |
| 18 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 19 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 20 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.21 | ND | ND | 0.02 | 0.44 | ND | ND | ND | ND |
| 21 | −0.12 | 0.03 | 0.08 | −0.09 | ND | 0.03 | −0.17 | −0.01 | 0.09 | 0.10 | −0.04 | 0.01 | −0.02 | −0.09 | −0.10 | 0.06 | −0.11 | −0.24 |
| 22 | −0.02 | 0.12 | 0.05 | −0.01 | −0.32 | 0.04 | −0.02 | 0.02 | 0.05 | 0.02 | −0.12 | 0.002 | 0.02 | −0.36 | −0.25 | 0.17 | −1.21 | −0.51 |
| 23 | −0.002 | 0.06 | 0.03 | ND | ND | ND | 0.02 | −0.10 | ND | 0.15 | −0.02 | 0.12 | −0.02 | 0.01 | −0.20 | 0.25 | −0.03 | 0.09 |
| 24 | −0.02 | 0.12 | ND | 0.06 | −0.17 | ND | 0.65 | −0.15 | 0.19 | 0.07 | 0.02 | 0.11 | −0.05 | −0.13 | −0.49 | −0.02 | −0.10 | −0.48 |
| 25 | −0.002 | 0.33 | 0.59 | 0.37 | −0.56 | 0.24 | −0.09 | 0.09 | 1.16 | 0.34 | −0.54 | −0.06 | 0.17 | −0.56 | −0.82 | 0.27 | −0.60 | −0.88 |
| 26 | −0.06 | 0.06 | ND | 0.15 | −0.20 | ND | 0.07 | −0.20 | 0.02 | 0.20 | −0.03 | ND | 0.05 | 0.01 | ND | 0.06 | −0.06 | 0.01 |
| 27 | −0.02 | 0.08 | 0.14 | 0.32 | −0.41 | 0.58 | 0.16 | −0.43 | 0.59 | 0.22 | −0.19 | −0.07 | 0.28 | −0.08 | −0.46 | 0.43 | −0.13 | −0.57 |
| 28 | −0.19 | 0.23 | 0.28 | 0.18 | −0.15 | 0.41 | 0.04 | 0.10 | 0.86 | 0.34 | −0.26 | −0.21 | 0.02 | −0.15 | −0.48 | 0.16 | −0.11 | −0.62 |
| Polarity: p | (−) *** | (+) *** | (+) *** | (+) ** | (−) ** | (+) *** | (+/−) | (+/−) | (+) *** | (+) *** | (−) *** | (−) * | (+) * | (−) *** | (−) *** | (+) *** | (−) ** | (−) ** |
| Absolute mobility: Mean±SD | 0.14 ± 0.13 | 0.31± 0.36 | 0.38 ± 0.29 | 0.38 ± 0.32 | 0.34 ± 0.29 | 0.24 ± 0.20 | 0.28 ± 0.26 | 0.32 ± 0.36 | 0.55 ± 0.55 | 0.34 ± 0.25 | 0.20 ± 0.22 | 0.16 ± 0.16 | 0.19 ± 0.19 | 0.22 ± 0.18 | 0.59 ± 0.33 | 0.33 ± 0.20 | 0.28 ± 0.30 | 0.59 ± 0.34 |
L, R and Bi: left, right and bilateral; Ma, Me: masseter, medial pterygoid; p, probability of random polarity by binomial test,
p<0.05;
p<0.01;
p<0.001.
shortening;
lengthening. ND: no data.
Figure 3.
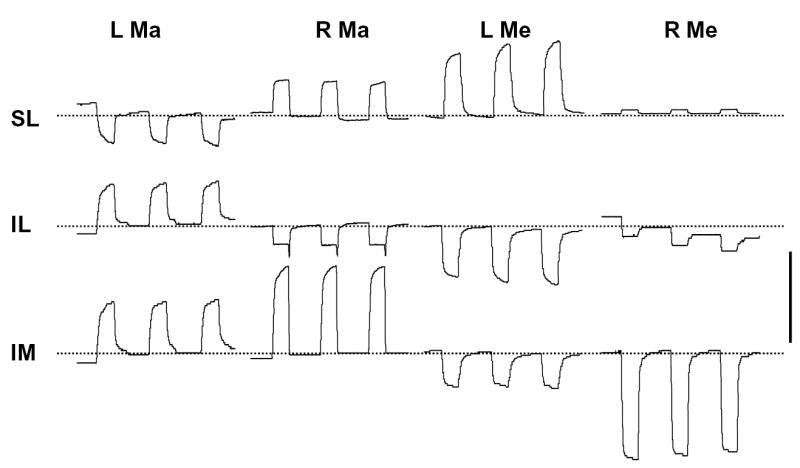
Displacement of the distraction site (right side) during muscle stimulation (from #28). L, left; R, right; Ma, masseter; Me, medial pterygoid; SL, superior-lateral; IL, inferior-lateral; IM, inferior-medial. Broken lines represent the approximate baseline. Although the baseline was not always stable, the relative amount of deflection remained consistent. An upward deflection represents lengthening and a downward deflection shortening. Scale bar, 0.4 mm.
Absolute mobility of the distraction site was consistently and significantly greater than the error level. During stimulation of left masseter and right medial pterygoid, the average mobility appeared to increase in the order SL, IL and IM, while during stimulation of right masseter and left medial pterygoid, the average mobility tended to decrease in that order (Table 3). However, ANOVA tests on animals having complete measurements from all three locations found that only the right medial pterygoid stimulation produced a significant location difference (p<0.001). It is clear from Table 3 that the reason for this is the strong displacement (0.59 mm shortening) produced at the IM location. Bilateral stimulation produced displacements that reflected the combined effects of individual muscles, but their magnitude could not be predicted by simple addition (same direction) or subtraction (opposite direction).
2. Displacement after bilateral osteotomy
Bilateral osteotomy was only performed on Group 2 pigs, in which displacement of the right and left osteotomy was measured by DVRTs and ultrasound crystals, respectively, during muscle stimulation. The results are shown in Table 4. Generally, similar displacement polarities to those of unilateral osteotomy were observed. However, there were more exceptions and in many cases the binomial test of polarity failed to reach statistical significance, especially for the left distraction site.
Table 4.
Bilateral osteotomy: displacement during muscle stimulation
| Right distraction site
|
Left distraction site
|
|||||
|---|---|---|---|---|---|---|
| Muscle stimulation | SL | IL | IM | SL | IL | IM |
| Left masseter: polarity | − (8/11†) | + (10/11)* | + (9/10)* | + (5/8) | − (10/11)* | + (7/9) |
| mobility (mm, mean ± SD) | 0.06±0.09 | 0.04±0.05 | 0.11±0.27 | 0.17±0.13 | 0.19±0.14 | 0.10±0.10 |
| Right masseter: polarity | + (9/12) | − (10/12)* | + (11/12)** | − (6/8) | + (7/10) | + (7/9) |
| mobility (mm, mean ± SD) | 0.13±0.14 | 0.11±0.12 | 0.12±0.12 | 0.04±0.04 | 0.05±0.08 | 0.08±0.15 |
| Left medial pterygoid: polarity | + (10/11)* | − (10/11)* | + (8/11) | + (6/8) | − (4/8) | − (9/10) |
| mobility (mm, mean ± SD) | 0.09±0.13 | 0.04±0.06 | 0.05±0.10 | 0.31±0.47 | 0.10±0.04 | 0.22±0.15 |
| Right medial pterygoid: polarity | + (6/11) | − (8/12) | − (11/12)** | + (8/10) | − (10/10)** | − (6/9) |
| mobility (mm, mean ± SD) | 0.05±0.05 | 0.04±0.04 | 0.23±0.16 | 0.10±0.09 | 0.11±0.16 | 0.15±0.26 |
fraction of pigs showing the indicated polarity (−, shortening, +, lengthening);
polarity by binomial test, p<0.05;
p<0.01.
Absolute mobility did not differ between right and left sides (paired t-test, p>0.05). Average displacements after bilateral osteotomy were generally smaller than after unilateral osteotomy. Although several of these decreases were statistically significant, because multiple t-tests were performed, this reduced mobility is best considered a trend.
3. Displacement after activation of the distractor
As presented elsewhere32, the actual opening of the distraction gap after activating the distractor 4 turns was less than 2 mm, averaging 1.5 mm. Because the groups did not differ, all measurements were combined for analysis of the right distraction site (Table 5). Compared to those before activation (Table 3), displacement polarities remained the same with the single exception of the medial location in response to left medial pterygoid stimulation, which became inconsistent. The magnitude of mobility tended to increase after distractor activation, although significance was reached only in a few of the multiple tests performed.
Table 5.
Distractor activation: displacement of the right distraction site during muscle stimulation
| Right distraction site displacement (mm)
|
|||
|---|---|---|---|
| Muscle stimulation | SL | IL | IM |
|
Left masseter: polarity
mobility (mm, mean ± SD) |
− (18/21†)**
0.36±0.35 |
+ (15/17)**
0.31±0.37 |
+ (20/21)***
0.46±0.47 |
|
Right masseter: polarity
mobility (mm, mean ± SD) |
+ (19/22)**
0.31±0.32 |
− (15/19)*
0.22±0.23 |
+ (21/23)***
0.28±0.25 |
|
Left medial pterygoid: polarity
mobility (mm, mean ± SD) |
+ (18/19)***
0.18±0.17 |
− (13/17)*
0.21±0.26 |
− (11/21)
0.17±0.20 |
|
Right medial pterygoid: polarity
mobility (mm, mean ± SD) |
+ (20/21)***
0.24±0.29 |
− (21/23)**
* 0.33±0.34 |
− (24/24)***
0.71±0.52 |
fraction of pigs showing the indicated polarity (−, shortening, +, lengthening);
binomial test of polarity, p<0.05;
p<0.01;
p<0.001.
DISCUSSION
The oblique osteotomy used in the present study was chosen to be comparable to a well-established pig model of mandibular distraction24–25, which itself mimics a standard human procedure1,4–5. While all osteotomies are probably mobile to the extent documented in the present study, other osteotomy designs and placements would be expected to have different mechanics and hence different displacement patterns.
Measurement of the osteotomy gap is technically difficult. Because of the high strain and large width of the gap, foil strain gauges cannot be used. In this study, we adapted ultrasound crystals and DVRTs to record across the distraction site by placing attached screws or pegs into drilled holes. These devices are somewhat vulnerable to interference from overlying soft tissues. An average displacement of 0.05 mm was recorded between crystals implanted on the intact left mandible during mastication. Given their original length (about 10 mm), this displacement equals approximately 5000 μɛ. This value is an order of magnitude larger than strain recorded from the mandibular body using strain gauges8, suggesting that there was interference from overlying tissues, most likely from masseter muscle contraction30.
Due to the greater reflection of muscles, devices across the distraction site should have had less overlying tissue than control crystals. Yet the distraction site (all three locations) had significantly higher mobility than the control crystals, proving that the mandibular DO site is mobilized by mastication despite fixation by a distractor appliance. No correlation was found between displacement magnitude and animal weight, which is assumed to be proportional to mandible size. This then suggests that mandible size is not an important determinant of stability at the DO site. In addition, the average magnitude of 0.3–0.4 mm is comparable to that estimated from miniplate fixation of mandibular angle fracture upon simulated functional loading23, suggesting this amount of instability is unavoidable when muscles contract. It should be noted that in a typical clinical situation, a soft diet would have been prescribed and vigorous muscle contraction avoided. Our hard diet results thus probably provide an indication of the maximum, not the average, displacement that could occur under clinical conditions.
Measuring displacement at three locations enables a three-dimensional consideration of how the osteotomy deforms. Comparison of the superior location with the inferior locations can reveal the existence of sagittal plane bending, while comparison of the medial location with the lateral locations can show bending in the transverse plane.
Left chews consistently exhibited superior lengthening and inferior shortening of the osteotomy. This pattern suggests sagittal plane bending with the distractor as the neutral axis. Basically, the superior edge of the mandible is under tension while the inferior border is compressed. The similar amount of shortening at both medial and lateral inferior locations suggests that there is no transverse bending. Shear movement is also not in evidence because such displacements would cause lengthening at all three locations. The documentation of sagittal bending with superior tension confirms that this deformation, commonly assumed to occur at the mandibular angle20 but not previously documented in vivo, does occur in response to loading.
However, a different pattern of displacement was seen for osteotomy-side (right) chewing. Right chews were rare, and displacements during right chews were variable, probably because pigs were unable to produce normal occlusal forces or movements on the osteotomized side. Relatively consistent shortening was observed at the SL and IM locations during right-side power strokes. The observed displacements suggest that chewing on the right simply approximated the bone fragments in compression.
Muscle stimulation confirmed the instability of the DO site. Significant displacement was only found during the contraction of jaw closers, consistent with the observation that the DO site was mainly mobilized during the power stroke of mastication. The similar magnitude of displacement during masseter and medial pterygoid stimulation to that of mastication further indicates that it is the jaw-closing muscle contraction with its resulting jaw movement and tooth contact that mobilizes the DO site. It is particularly interesting that this effect was seen even after the partial reflection of the surgery-side muscles. The implication is that relatively little muscle force was required to displace the osteotomy. The muscular effects of mandibular distraction may include either atrophy33 or hypertrophy34, but even in the case of atrophy, the muscles may still be powerful enough to distort the osteotomy site.
Muscle stimulations, however, did not unambiguously explain the displacement patterns of mastication. For left chews, it is known that the predominant muscles for the power strokes are left masseter and medial pterygoid and right temporalis9. Unfortunately, the temporalis was not examined by stimulation. Left medial pterygoid stimulation did mimic the pattern of left chews, but the left masseter caused an opposite pattern. These two muscles have opposite rotatory effects in the transverse plane, which presumably causes their opposite displacement patterns. The left masseter rotates the lower portion of the large mandibular fragment laterally while the medial pterygoid rotates it medially. However, during mastication these muscles co-contract, which should cancel out the rotatory components. There is no a priori reason why the left medial pterygoid should dominate the left masseter in deforming the osteotomy. More likely, the muscle we did not examine, the right temporalis, is the critical element. The right temporalis would directly mobilize the small fragment during mastication by pulling posteriorly on the coronoid process. It is reasonable to speculate that the temporalis rotated the small fragment in the sagittal plane, resulting in superior separation and inferior approximation of the DO site.
For the right-side power stroke, the predominant muscles are the right masseter and medial pterygoid and the left temporalis. Of particular interest is the superior location, which generally shortened during right chews but lengthened during both right masseter and medial pterygoid stimulations. Again, the temporalis may play a role. As the right masseter and medial pterygoid brought the small fragment upward and forward, the left temporalis would pull upward and backward on the large fragment, resulting in approximation of the two fragments at all locations.
Both bilateral and unilateral mandibular distractions are common in pediatric clinical practice, the former for relatively symmetrical disorders such as Treacher Collins syndrome and the latter for more asymmetrical problems such as hemifacial microsomia. This study compared the procedures by measuring the displacement during muscle stimulation before and after a second osteotomy. Because the stimulating electrodes were kept in place (for the most part) during the second osteotomy, the muscle contractions were highly reproducible, enabling a good comparison. Compared to unilateral osteotomy, bilateral osteotomy tended to decrease mobility at the DO site. This is not surprising. Bilateral osteotomy produces three (instead of two) mandibular fragments of the mandible and isolates the symphysis, a condition that prevents force from being transmitted from side to side. Polarity became more variable, presumably because the second osteotomy allowed additional mechanics. Thus, increasing the number of osteotomies may cause smaller but more complex deformations.
Activation of the distractor had little effect on either polarity or magnitude of displacement during muscle stimulations. Thus the basic mechanics are unchanged by separating the fragments. The slightly increase magnitudes of displacement presumably reflect the greater compliance of the activated appliance, and might have been larger had the distractor been opened fully. However, it is not clear that this procedure actually mimicked a true distraction treatment. Factors during actual distraction such as jaw deviation, fibrous union between fragments and lateral occlusal relationships can all affect masticatory mechanics and will require investigation of chronic DO models.
In conclusion, the results of this study confirm that the mandibular distraction osteotomy site is not completely stabilized by a distractor. The contraction of masticatory muscles, especially jaw closers, and consequent jaw movement and tooth contact strain the DO site, leading to significant interfragmentary displacement.
Acknowledgments
Funded by PHS award DE 14336. We thank Dr. Zi-Jun Liu and Mr. Frank Starr for their assistance with experiments. We also thank Synthes Maxillofacial, KLS-Martin and Stryker Instruments for their donations of materials and product support.
References
- 1.McCarthy JG, Schreiber J, Karp N, et al. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1. [PubMed] [Google Scholar]
- 2.Polley JW, Figueroa AA. Management of severe maxillary deficiency in childhood and adolescence through distraction osteogenesis with an external, adjustable, rigid distraction device. J Craniofac Surg. 1997;8:181. doi: 10.1097/00001665-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hollier LH, Kim JH, Grayson B, et al. Mandibular growth after distraction in patients under 48 months of age. Plast Reconstr Surg. 1999;103:1361. doi: 10.1097/00006534-199904050-00004. [DOI] [PubMed] [Google Scholar]
- 4.Molina F, Ortiz Monasterio F. Mandibular elongation and remodeling by distraction: A farewell to major osteotomies. Plast Reconstr Surg. 1995;96:825. [PubMed] [Google Scholar]
- 5.McCarthy JG, Stelnicki EJ, Mehrara BJ, et al. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001;107:1812. doi: 10.1097/00006534-200106000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Rel Res. 1990;250:8. [PubMed] [Google Scholar]
- 7.Hylander WL. Mandibular function in galago crassicaudatus and macaca fascicularis: An in vivo approach to stress analysis of the mandible. J Morph. 1979;159:253. doi: 10.1002/jmor.1051590208. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZJ, Herring SW. Bone surface strains and internal bony pressures at the jaw joint of the miniature pig during masticatory muscle contraction. Arch Oral Biol. 2000;45:95. doi: 10.1016/s0003-9969(99)00127-2. [DOI] [PubMed] [Google Scholar]
- 9.Herring SW, Rafferty KL, Liu ZJ, et al. Jaw muscles and the skull in mammals: The biomechanics of mastication. Comp Biochem Physiol A Mol Integr Physiol. 2001;131:207. doi: 10.1016/s1095-6433(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 10.Hylander WL, Crompton AW. Jaw movements and patterns of mandibular bone strain during mastication in the monkey macaca fascicularis. Arch Oral Biol. 1986;31:841. doi: 10.1016/0003-9969(86)90139-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Langenbach GE, Hannam AG, et al. Mass properties of the pig mandible. J Dent Res. 2001;80:327. doi: 10.1177/00220345010800010601. [DOI] [PubMed] [Google Scholar]
- 12.Buckwalter JA. Effects of early motion on healing of musculoskeletal tissues. Hand Clin. 1996;12:13. [PubMed] [Google Scholar]
- 13.Goodship AE, Cunningham JL, Kenwright J. Strain rate and timing of stimulation in mechanical modulation of fracture healing. Clin Orthop Rel Res. 1998;355S:S105. doi: 10.1097/00003086-199810001-00012. [DOI] [PubMed] [Google Scholar]
- 14.Augat P, Burger J, Schorlemmer S, et al. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J Orthop Res. 2003;21:1011. doi: 10.1016/S0736-0266(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 15.Aro H: Distraction of the craniofacial skeleton. New York, NY, Springer, 1999, p 20
- 16.Cope JB, Samchukov ML, Cherkashin AM. Mandibular distraction osteogenesis: A historic perspective and future directions. Amer J Ortho Dentofac Orthop. 1999;115:448. doi: 10.1016/s0889-5406(99)70266-0. [DOI] [PubMed] [Google Scholar]
- 17.Cope JB, Samchukov ML. Regenerate bone formation and remodeling during mandibular osteodistraction. Angle Orthod. 2000;70:99. doi: 10.1043/0003-3219(2000)070<0099:RBFARD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.King GJ, Liu ZJ, Wang LL, et al. Effect of distraction rate and consolidation period on bone density following mandibular osteodistraction in rats. Arch Oral Biol. 2003;48:299. doi: 10.1016/s0003-9969(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 19.Del Santo M, Jr, Guerrero CA, Buschang PH, et al. Long-term skeletal and dental effects of mandibular symphyseal distraction osteogenesis. Amer J Ortho Dentofac Orthop. 2000;118:485. doi: 10.1067/mod.2000.109887. [DOI] [PubMed] [Google Scholar]
- 20.Shetty V, McBrearty D, Fourney M, et al. Fracture line stability as a function of the internal fixation system: An in vitro comparison using a mandibular angle fracture model. J Oral Maxillofac Surg. 1995;53:791. doi: 10.1016/0278-2391(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 21.Choi BH, Yoo JH, Kim KN, et al. Stability testing of a two miniplate fixation technique for mandibular angle fractures. An in vitro study J Craniomaxillofac Surg. 1995;23:123. doi: 10.1016/s1010-5182(05)80460-3. [DOI] [PubMed] [Google Scholar]
- 22.Feller KU, Schneider M, Hlawitschka M, et al. Analysis of complications in fractures of the mandibular angle--a study with finite element computation and evaluation of data of 277 patients. J Craniomaxillofac Surg. 2003;31:290. doi: 10.1016/s1010-5182(03)00015-5. [DOI] [PubMed] [Google Scholar]
- 23.Schierle HP, Schmelzeisen R, Rahn B, et al. One- or two-plate fixation of mandibular angle fractures? J Craniomaxillofac Surg. 1997;25:162. doi: 10.1016/s1010-5182(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 24.Glowacki J, Shusterman EM, Troulis M, et al. Distraction osteogenesis of the porcine mandible: Histomorphometric evaluation of bone. Plast Reconstr Surg. 2004;113:566. doi: 10.1097/01.PRS.0000101061.99577.09. [DOI] [PubMed] [Google Scholar]
- 25.Kaban LB, Thurmuller P, Troulis MJ, et al. Correlation of biomechanical stiffness with plain radiographic and ultrasound data in an experimental mandibular distraction wound. Int J Oral Maxillofac Surg. 2003;32:296. doi: 10.1054/ijom.2002.0380. [DOI] [PubMed] [Google Scholar]
- 26.Ström D, Holm S, Clemensson E, et al. Gross anatomy of the mandibular joint and masticatory muscles in the domestic pig (sus scrofa) Arch Oral Biol. 1986;31:763. doi: 10.1016/0003-9969(86)90009-9. [DOI] [PubMed] [Google Scholar]
- 27.Herring SW. The dynamics of mastication in pigs. Arch Oral Biol. 1976;21:473. doi: 10.1016/0003-9969(76)90105-9. [DOI] [PubMed] [Google Scholar]
- 28.Herring SW: Temporomandibular disorders and related pain conditions. Seattle, WA, IASP Press, 1995, p 323
- 29.Huang X, Zhang G, Herring SW. Age changes in mastication in the pig. J Comp Biochem Physiol. 1994;107A:647. doi: 10.1016/0300-9629(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 30.Herring SW, Sun Z, Egbert MA, et al: Is distraction the only motion permitted at the osteotomy site? Biological Mechanisms of Tooth Movement and Craniofacial Adaptation, New York, 2004, p 31
- 31.Rafferty KL, Sun Z, Baird E, et al: Mandibular mechanics following osteotomy and distraction appliance placement: II. Bone strain on the body and condylar neck. J Oral Maxillofac Surg 2005, In revision [DOI] [PMC free article] [PubMed]
- 32.Sun Z, Rafferty KL, Egbert MA, et al: Mandibular osteotomy site separation during acute distraction. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation, New York, 2004, p 53
- 33.Fisher E, Staffenberg DA, McCarthy JG, et al. Histopathologic and biochemical changes in the muscles affected by distraction osteogenesis of the mandible. Plast Reconstr Surg. 1997;99:366. doi: 10.1097/00006534-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Mackool RJ, Hopper RA, Grayson BH, et al. Volumetric change of the medial pterygoid following distraction osteogenesis of the mandible: An example of the associated soft-tissue changes. Plast Reconstr Surg. 2003;111:1804. doi: 10.1097/01.PRS.0000055431.19215.0A. [DOI] [PubMed] [Google Scholar]


