Abstract
There is increasing recognition at both the international and national level of the disease burden attributed to psychiatric disorders, yet little is known about how much of this burden is or could be averted by current or scaled-up implementation of effective interventions. In addition, little is known about the costs and cost-effectiveness of such interventions in most regions of the world, even though such information is of direct relevance to increased investment and service development. This research report provides an overview of the mental health component of the World Health Organization's CHOICE project, the aim of which is to generate cost-effectiveness evidence for a large number of interventions for leading contributors to disease burden in a range of geographical and epidemiological settings around the world. To date, expected costs (expressed in international dollars) and effects (measured in terms of disability adjusted life years, DALYs) of key pharmacological and psychosocial interventions have been modelled for schizophrenia, bipolar disorder, depression and panic disorder. The results of this analysis indicate that the most efficient interventions for common mental disorders (depression and panic disorder) can be considered very cost-effective (each DALY averted costs less than one year of average per capita income), while community-based interventions for more severe mental disorders using older antipsychotic and mood stabilising drugs meet the criterion for being cost-effective (each DALY averted costs less than three times the average annual income). These findings provide relevant new information to health policy makers regarding the relative value of investing in psychiatric care, and in so doing may help to remove one of many remaining barriers to a more appropriate public health response to the burden of these conditions.
Keywords: Costs, cost-effectiveness, DALYs, evidence-based treatment, mental health
Recent epidemiological research has clearly demonstrated the considerable (and previously underestimated) burden that mental disorders impose on individuals, communities, and health services throughout the world (1). Using a summary measure of population health, called the disability-adjusted life year or DALY (a time-based measure that combines in a single indicator years of life lost from premature death and years of life lived with a disability), the most recent estimates from the Global Burden of Disease study indicate that neuropsychiatric disorders contribute to more than 10% of lost years of healthy life and over 30% of all years lived with disability (1). The study showed in particular that unipolar depressive disorders place an enormous burden on society, in fact ranked as the fourth leading cause of burden among all diseases, accounting for over 50 million lost years of healthy life worldwide (2).
To date, however, only a limited connection has been made between DALYs and the generation of cost-effectiveness evidence, despite the fact that such a link was a central aim of the Global Burden of Disease study. The link is needed because DALYs are not in themselves sufficient as a mechanism for resource allocation and priority-setting in health care. A disease can place a considerable burden on a population but, if appropriate strategies or interventions to reduce this burden are absent or extremely expensive in relation to the health gains achieved, large-scale investment would be considered misplaced (since scarce resources could be more efficiently channelled to other burdensome conditions for which cost-effective responses are available). In other words, the size of the attributable burden alone is not sufficient to guide action. For prioritysetting and resource allocation, a more pertinent question is to ask what is the avertable burden of a particular disease arising from the use of an evidence-based set of interventions and what is the relative cost of their implementation in the target population. Such an analysis can reveal the most efficient response to the attributable burden of a particular disease.
The last two decades have seen an ever-increasing interest in, and demand for, economic analysis of mental health care and policy, fuelled by government concerns about rises in health care expenditures (3). Considerations of cost and cost-effectiveness enter into health care reform processes, priority-setting exercises within and across health programmes, and regulatory decisions concerning drug approval or pricing. Despite the need for cost-effectiveness evidence, however, there remains a relative paucity of completed mental health economic evaluations from both developed and developing countries (4). The preponderance of completed economic evaluations in mental health care have been concerned with specific treatment modalities for psychoses and affective disorders, in particular the cost-effectiveness of different psychotropic medications and, more recently, various psychotherapeutic approaches to the management of these psychiatric disor- ders (5,6). Many mental health economic studies undertaken to date suffer from sub-optimal design, an unclear cost perspective, or inadequate power. There is a consequent requirement to derive more appropriately powered and generalized estimates of both the costs and relative cost-effectiveness of interventions in order to usefully inform mental health policy and planning, both at the national and international level.
Development of such an economic evidence base in mental health can be achieved in two ways. Preferably, it would be generated on the back of additional empirical studies in a range of socioeconomic settings (particularly developing countries, where current evidence is most scarce). Well-designed and sufficiently-powered economic evaluations of mental health interventions are certainly needed and valuable, but they are also difficult, time-consuming and expensive to carry out (as well as having limited application beyond the immediate confines of the study location), meaning that it is very unlikely that a sufficient evidence base will be generated in this incremental and relativist manner, even within the next 10 years. Alternatively, and more immediately, the current information vacuum can be filled via appropriate disease modelling of best available existing data concerning the expected costs and effects of interventions in these different settings.
The danger of the latter, more universalist approach lies in the inevitable assumptions that are required when basing cost-effectiveness estimates on a variety of sources from different places, while the obvious attraction is that policy-relevant results can be generated rather quickly and cheaply. Over the longer-term, these two approaches can in fact be considered complementary - empirical studies feed into initial and revised modelling exercises, while modelling studies synthesise and may even stimulate empirical research studies - but this should not detract from the shorter-term need in most regions of the world to bring cost-effectiveness arguments into play when arguing for an increased level of resource investment to and prioritisation for mental health service development.
METHODS
Selection of psychiatric disorders and interventions
Three key criteria guided the choice of psychiatric disorders to which this sectoral approach to cost-effectiveness analysis has been applied: public health burden and importance; the availability of effective and potentially cost-effective interventions; and the availability of data on epidemiology, clinical effectiveness, resource utilisation and costs. Concerning the first of these criteria, schizophrenia, bipolar disorder, (unipolar) depression and obsessive-compulsive disorder (OCD) all appear in the ten leading causes of disability worldwide (1). For each of these burdensome conditions, a set of personal interventions covering key psychopharmacological and psychosocial treatments was identified and reviewed; international evidence for the effectiveness of specific health care interventions was sufficiently robust for all of the above conditions except OCD (as a result of which, panic disorder was selected as the index condition covering anxiety disorders). A full list of interventions subjected to economic analysis is given in Table 1.
Table 1.
Interventions for psychiatric conditions subjected to economic analysis
| Disorder | Intervention |
|---|---|
| Schizophrenia | Older (neuroleptic) antipsychotic drug |
| Treatment setting: hospital outpatient | Newer (‘atypical’) antipsychotic drug |
| Treatment coverage (target): 80% | Older antipsychotic drug + psychosocial treatment |
| Newer antipsychotic drug + psychosocial treatment | |
| Bipolar disorder | Older mood stabilising drug |
| Treatment setting: hospital outpatient | Newer mood stabilising drug |
| Treatment coverage (target): 50% | Older mood stabilising drug + psychosocial treatment |
| Newer mood stabilising drug + psychosocial treatment | |
| Depression | Episodic treatment |
| Treatment setting: primary health care | Older (tricyclic) antidepressant drug |
| Treatment coverage (target): 50% | Newer antidepressant drug |
| Psychosocial treatment | |
| Older antidepressant drug + psychosocial treatment | |
| Newer antidepressant drug + psychosocial treatment | |
| Maintenance treatment | |
| Older antidepressant drug + psychosocial treatment | |
| Newer antidepressant drug + psychosocial treatment | |
| Panic disorder | Benzodiazepines |
| Treatment setting: primary health care | Older (tricyclic) antidepressant drug |
| Treatment coverage (target): 50% | Newer antidepressant drug |
| Psychosocial treatment | |
| Older antidepressant drug + psychosocial treatment | |
| Newer antidepressant drug + psychosocial treatment | |
WHO guidelines on cost-effectiveness analysis
The World Health Organization (WHO), through its CHOICE work programme (CHOosing Interventions that are Cost-Effective), proposes a form of cost-effectiveness analysis that provides policy makers with a set of results that are generalisable across settings (7). It does this by evaluating the costs and effectiveness of new and existing interventions compared to the starting point of doing none of the current interventions. Importantly, the use of such a common reference removes the constraint that the current intervention mix must be continued and eliminates differences in starting points, which make the results of incremental analysis difficult to transfer across settings. Only one constraint remains, the budget, which allows simple decision rules to be developed based on the calculated cost-effectiveness ratios. Cost-effectiveness results can be used to define three broad sets of interventions ??those which improve population health a great deal for a given set of resources; those which are not efficient ways to improve health; and those which are in between. This information enters the policy debate to be weighed against the impact of different intervention mixes on other objectives such as reducing health inequalities and responding to the legitimate expectations of populations. Policy makers can then assess if it is in the country's best interest to retain the current portfolio of interventions or modify it.
Key steps in the application of sectoral cost-effectiveness analysis
The application of generalised cost-effectiveness analysis in a systematic and standardised manner involves a number of key analytical steps that touch upon a diverse yet inter-related set of disciplinary areas, including demography, epidemiology, clinical effectiveness, cost analysis and health economics.
Step 1: Construct a profile of observed epidemiology. WHO-CHOICE pursues a population-based, epidemiologically- based approach to cost-effectiveness analysis. Accordingly, for the disorder and population in question, the first analytical step is to generate a profile or model of the prevailing epidemiological situation. The standard reference point for such a profile is the latest version of the Global Burden of Disease study (1), which provides empirically-based but internally consistent estimates of the incidence, prevalence, remission and case-fatality for all leading causes of disease burden globally.
Step 2: Construct natural history models. A particular feature of WHO-CHOICE is its use of a no treatment scenario as a basis for comparing the relative costs and consequences of different health interventions (7). For psychiatric conditions, natural history models were used (rather than a process of back-adjustment from existing effective coverage of interventions in the population). However, for some mental disorders and in certain regions of the world, it should be noted that at a population-wide level the current situation is in fact a very good approximation of the no treatment scenario (typically because so little intervention is taking place).
Step 3. Calculate population-level intervention effectiveness. Intervention effectiveness was determined via a state transition population model, which traces the development of a regional population taking into account births, deaths and the disease in question. Key transition rates, each expressed in terms of number of events per year at risk, include the incidence of the disorder in the population, case-fatality and remission. In addition, a disability weight is specified (on a 0-1 scale) for time spent in different states of (ill-)health. Two epidemiological situations are modelled over a lifetime analytic horizon, to give the total number of healthy years lived by the population: a) a counterfactual epidemiological situation representing the natural history of disease (no interventions in operation); and b) the epidemiological situation reflecting the population-level impact of each specified intervention (such as reduced illness duration resulting from use of an antidepressant drug), implemented for a period of 10 years. The difference between these two simulations represents the population-level health gain (measured in DALYs averted) due to the implementation of the intervention, relative to the situation of doing nothing. DALYs averted are discounted (at 3%) but not age-weighed in the base case analysis reported below.
Changes in parameters resulting from successful intervention need to reflect effectiveness rather than efficacy, so as a minimum it is necessary to make adjustments for expected intervention coverage rates at the population level as well as patient-level adherence to treatment. Sources of data for intervention efficacy and effectiveness included meta-analyses, systematic reviews and individual clinical trials reported in the international literature. Estimation of treatment effectiveness for specific disorders are reported in detail elsewhere (8-10).
Step 4: Construct resource utilisation and cost profile(s) for each intervention. An 'ingredients' approach to the costing of health interventions has been used, which requires separate identification and valuation of the quantity of resource inputs needed (such as numbers of health personnel) and the price or unit cost of those resource inputs (such as the salary of a health professional). For patient-level resource quantities (e.g. hospital inpatient days, outpatient visits, medications, laboratory tests, etc.), information sources include data from economic evaluations and also a multinational Delphi consensus study specifically undertaken for WHO-CHOICE and neuropsychiatric disorders (11).
Unit costs associated with these items of service use have been calculated for each World Bank region, based on an econometric analysis of a multinational dataset of hospital costs, using gross domestic product per capita (plus other explanatory variables) to predict unit costs in different regions (12). In addition, programme costs were computed, which are resources that are used in the production of an intervention at a level above that of the patient or providing facility, including central planning, policy and administration functions, as well as resources devoted to training health providers. All baseline analysis costs for the 10-year implementation period were discounted at 3% and expressed in international dollars (I$), which adjust for differences in the relative price and purchasing power of countries and thereby facilitate comparison across regions. That is, one international dollar buys the same quantity of health care resources in China or India as it does in the United States of America.
Step 5: Cost-effectiveness analysis (including uncertainty). The assembly of the various data components described above provides the building blocks for analysis of the costs and effects of a mental health care intervention. Once input values for these data components have been finalised, summary results for population-level costs, effectiveness and cost-effectiveness can be generated, including the comparative efficiency of specified interventions, expressed as average and incremental cost-effectiveness ratios (CERs) of I$ per DALY averted. In common with any robust economic evaluation, it is important to provide an indication of the uncertainty around point estimates of cost, effect or CER. Firstly, a series of one-way sensitivity analyses that assess the impact on final results of discounting and age-weighing can be performed. Secondly, best and worse case scenarios incorporating upper and lower values for key drivers of cost (unit price of drugs and health care services, the proportion of cases using secondary services) and treatment effectiveness (efficacy and adherence) can be generated. In addition, a stochastic uncertainty analysis was performed of the probability that individual interventions - including both current and new interventions - would be selected as a cost-effective use of resources given a specified budget constraint (7).
RESULTS
Estimation methods, baseline results and uncertainty analyses for individual conditions are reported in detail elsewhere, either by WHO epidemiological sub-region (8, 9) or by World Bank region (10). Here, summary estimates of the population-level effects (measured in DALYs averted) and cost-effectiveness of each intervention are presented by World Bank region for the four psychiatric disorders selected for analysis (Tables 2-3).
Table 2.
Population-level intervention effects (DALYs averted per year per one million population)
| World Bank Region | |||||||
|---|---|---|---|---|---|---|---|
| Sub-Saharan | Latin America | Middle East | Europe | South | East Asia | ||
| Africa | & Caribbean | & North Africa | & Central Asia | Asia | & Pacific | ||
| Total population (million) | 640 | 502 | 482 | 462 | 1,242 | 1,827 | |
| Coverage | |||||||
| Schizophrenia1 | |||||||
| Older (neuroleptic) antipsychotic drug | 80% | 149 | 219 | 214 | 254 | 177 | 231 |
| Newer antipsychotic drug | 80% | 160 | 235 | 230 | 273 | 190 | 248 |
| Older antipsychotic drug + | 80% | 254 | 373 | 364 | 353 | 300 | 392 |
| psychosocial treatment | |||||||
| Newer antipsychotic drug + | 80% | 261 | 383 | 373 | 364 | 308 | 403 |
| psychosocial treatment | |||||||
| Bipolar disorder1 | |||||||
| Older mood stabilising drug (lithium) | 50% | 292 | 336 | 296 | 381 | 319 | 389 |
| Newer mood stabilising drug (valproate) | 50% | 211 | 300 | 273 | 331 | 278 | 351 |
| Older mood stabilising drug + | 50% | 312 | 365 | 322 | 413 | 346 | 422 |
| psychosocial treatment | |||||||
| Newer mood stabilising drug + | 50% | 232 | 330 | 300 | 365 | 306 | 386 |
| psychosocial treatment | |||||||
| Depression | |||||||
| Episodic treatment: older | 50% | 599 | 995 | 920 | 874 | 987 | 891 |
| antidepressant drug (TCA) | |||||||
| Episodic treatment: newer | 50% | 632 | 1,049 | 971 | 925 | 1,042 | 941 |
| antidepressant drug (SSRI) | |||||||
| Episodic psychosocial treatment | 50% | 624 | 1,036 | 958 | 936 | 1,028 | 927 |
| Episodic psychosocial treatment + | 50% | 745 | 1,237 | 1,144 | 1,100 | 1,228 | 1,107 |
| older antidepressant | |||||||
| Episodic psychosocial treatment + | 50% | 745 | 1,237 | 1,144 | 1,100 | 1,228 | 1,107 |
| newer antidepressant | |||||||
| Maintenance psychosocial treatment + | 50% | 1,174 | 1,953 | 1,806 | 1,789 | 1,937 | 1,747 |
| older antidepressant | |||||||
| Maintenance psychosocial treatment + | 50% | 1,174 | 1,953 | 1,806 | 1,789 | 1,937 | 1,747 |
| newer antidepressant | |||||||
| Panic disorder | |||||||
| Anxiolytic drug (benzodiazepine) | 50% | 144 | 182 | 170 | 183 | 168 | 195 |
| Older antidepressant | 50% | 232 | 290 | 272 | 290 | 269 | 312 |
| drug (TCA) | |||||||
| Newer antidepressant drug | 50% | 245 | 307 | 287 | 307 | 284 | 330 |
| (SSRI; generic) | |||||||
| Psychosocial treatment (CBT) | 50% | 233 | 292 | 273 | 292 | 270 | 313 |
| Older antidepressant + | 50% | 262 | 329 | 308 | 329 | 304 | 353 |
| psychosocial treatment | |||||||
| Newer antidepressant + | 50% | 276 | 346 | 324 | 346 | 320 | 372 |
| psychosocial treatment | |||||||
Results for community-based service model presented here only (hospital-based service model not shown); CBT - cognitive behavioural therapy; DALYs - disability- adjusted life years; SSRI - selective serotonin reuptake inhibitor; TCA - tricyclic antidepressant
Table 3.
Average cost-effectiveness of interventions at specified levels of coverage (I$ per DALY averted)
| World Bank Region | |||||||
|---|---|---|---|---|---|---|---|
| Sub-Saharan | Latin America | Middle East | Europe | South | East Asia | ||
| Africa | & Caribbean | & North Africa | & Central Asia | Asia | & Pacific | ||
| Total population (million) | 640 | 502 | 482 | 462 | 1,242 | 1,827 | |
| Coverage | |||||||
| Schizophrenia1 | |||||||
| Older (neuroleptic) antipsychotic drug | 80% | 5,202 | 13,369 | 6,882 | 12,260 | 4,482 | 8,760 |
| Newer antipsychotic drug | 80% | 18,497 | 26,199 | 19,594 | 25,693 | 17,991 | 22,010 |
| Older antipsychotic drug + | 80% | 3,314 | 8,993 | 4,511 | 10,089 | 2,887 | 5,814 |
| psychosocial treatment | |||||||
| Newer antipsychotic drug + | 80% | 11,669 | 17,352 | 12,562 | 20,627 | 11,354 | 14,281 |
| psychosocial treatment | |||||||
| Bipolar disorder1 | |||||||
| Older mood stabilising drug (lithium) | 50% | 3,025 | 8,706 | 6,122 | 8,051 | 3,302 | 6,103 |
| Newer mood stabilising drug (valproate) | 50% | 4,829 | 10,074 | 6,935 | 9,620 | 4,422 | 7,230 |
| Older mood stabilising drug + | 50% | 2,903 | 7,785 | 5,492 | 7,233 | 3,136 | 5,524 |
| psychosocial treatment | |||||||
| Newer mood stabilising drug + | 50% | 4,520 | 8,988 | 6,222 | 8,607 | 4,147 | 6,530 |
| psychosocial treatment | |||||||
| Depression | |||||||
| Episodic treatment: older | 50% | 1,026 | 2,219 | 1,193 | 2,178 | 924 | 1,469 |
| antidepressant drug (TCA) | |||||||
| Episodic treatment: newer | 50% | 1,396 | 2,518 | 1,531 | 2,526 | 1,290 | 1,801 |
| antidepressant drug (SSRI) | |||||||
| Episodic psychosocial treatment | 50% | 1,384 | 2,726 | 1,499 | 2,494 | 1,205 | 1,787 |
| Episodic psychosocial treatment + | 50% | 1,416 | 2,595 | 1,487 | 2,421 | 1,256 | 1,738 |
| older antidepressant | |||||||
| Episodic psychosocial treatment + | 50% | 1,819 | 2,982 | 1,866 | 2,860 | 1,641 | 2,125 |
| newer antidepressant | |||||||
| Maintenance psychosocial treatment + | 50% | 1,706 | 2,935 | 1,721 | 2,589 | 1,547 | 1,968 |
| older antidepressant | |||||||
| Maintenance psychosocial treatment + | 50% | 2,245 | 3,460 | 2,229 | 3,162 | 2,072 | 2,487 |
| newer antidepressant | |||||||
| Panic disorder | |||||||
| Anxiolytic drug (benzodiazepine) | 50% | 1,277 | 1,853 | 1,237 | 1,748 | 997 | 1,332 |
| Older antidepressant | 50% | 1,013 | 1,378 | 984 | 1,328 | 842 | 1,057 |
| drug (TCA) | |||||||
| Newer antidepressant drug | 50% | 1,174 | 1,519 | 1,135 | 1,481 | 1,010 | 1,219 |
| (SSRI; generic) | |||||||
| Psychosocial treatment (CBT) | 50% | 1,276 | 1,666 | 1,145 | 1,702 | 970 | 1,271 |
| Older antidepressant + | 50% | 1,583 | 1,942 | 1,440 | 1,983 | 1,303 | 1,584 |
| psychosocial treatment | |||||||
| Newer antidepressant + | 50% | 1,722 | 2,061 | 1,570 | 2,121 | 1,441 | 1,720 |
| psychosocial treatment | |||||||
Results for community-based service model presented here only (hospital-based service model not shown); CBT - cognitive behavioural therapy; DALYs - disability- adjusted life years; SSRI - selective serotonin reuptake inhibitor; TCA - tricyclic antidepressant
Population-level effectiveness of interventions
Even at a treatment coverage rate of 80% (i.e. four out of every five cases), the impact of pharmacological treatments for schizophrenia - whether with older neuroleptics or newer antipsychotic drugs - is modest (150-250 DALYs averted annually per one million population), reflecting the fact that interventions do not reduce the incidence or duration of the disease so much as making a difference to the day-to-day functioning of treated patients (approximately a 25% improvement over no treatment when treated with antipsychotic drugs alone, or closer to 45% when given adjuvant psychosocial treatment in addition) (13,14). However, it needs to be emphasised that the full consequences of this often-catastrophic disease (on family life and the ability to be productive) are not adequately captured by the DALY metric. The addition of psychosocial treatment to pharmacotherapy is projected to have a far more pronounced benefit than switching from older to newer antipsychotic drugs (Table 2). Such a trend is also apparent for bipolar disorder, but with the added projection that, due to its established impact on reducing suicide, the older mood stabilising drug lithium is expected to generate more health gain in the population than newer mood stabilising drugs such as valproate (9). At a target coverage rate of 50%, health gains for the treatment of bipolar disorder and panic disorder are both in the range 150-400 DALYs averted annually per one million population, whereas episodic treatment of depression with antidepressants and/or psychotherapy generate much larger gains (600-1,200 DALYs averted), in large part due to the higher prevalence of this disorder in the population. Proactive, maintenance depression treatment has higher health returns still (1,200-1,900 DALYs averted), because in this scenario a significant proportion of recurrent depressive episodes would be prevented (8).
Intervention costs
The total cost per capita of community-based outpatient treatment with first generation antipsychotic or mood stabilising drugs, including all patient-level resource needs as well as infrastructural support, ranged from I$ 0.80-1.10 in Sub-Saharan Africa and South Asia to I$ 3 in the Latin America and Caribbean, and Europe and Central Asia regions (equivalent patient costs per year, I$ 300-550 and I$ 800-1,500 respectively). The cost per capita for second generation (atypical) antipsychotic drugs still under patent is much higher (I$ 3-7). By contrast, some of the newer antidepressant drugs (selective serotonin reuptake inhibitors, SSRIs) are now off patent and accordingly their use in treating depression and panic disorder was costed at their generic, non-branded price. The patient-level cost of treating a six-month episode of depression ranged from as little as I$ 50 (older antidepressants in Sub-Saharan Africa or South Asia) to I$ 150-200 (newer antidepressants in combination with brief psychotherapy in Latin America and Caribbean, and Europe and Central Asia). Total annual costs for all incident depressive episodes in receipt of treatment, including training and other program-level costs, were as much as I$ 2.50-6.50 per capita for a maintenance treatment program, three or four times more costly than episodic treatment with older antidepressant drugs only.
Cost-effectiveness of interventions
Compared to the epidemiological situation of no treatment (natural history), the most cost-effective strategy for averting the burden of psychosis and severe affective disorders is expected to be a combined intervention of first generation antipsychotic or mood stabilising drugs with adjuvant psychosocial treatment delivered via a communitybased outpatient service model, with a cost-effectiveness ratio close to I$ 3,000 in Sub-Saharan Africa and South Asia, rising to I$ 8,000-10,000 in middle-income regions (Table 3). Currently, the high acquisition price of secondgeneration antipsychotic drugs makes their use in developing regions questionable on efficiency grounds alone, although this situation stands to change as these drugs come off patent. By contrast, evidence indicates that the relatively modest additional cost of adjuvant psychosocial treatment reaps significant health gains, thereby making such a combined strategy for schizophrenia and bipolar disorder treatment more cost-effective than pharmacotherapy alone.
For more common mental disorders treated in primary care settings (depressive and anxiety disorders), the single most cost-effective strategy is the scaled-up use of older antidepressants (due to their lower cost but similar efficacy to newer antidepressants). However, as the price margin between older and generic newer antidepressants continues to diminish, generic SSRIs can be expected to be at least as cost-effective and may therefore constitute the treatment of choice in the future. Since depression is so commonly a recurring condition, there are also good grounds for thinking that proactive care management, including long-term maintenance treatment with antidepressant drugs, represents a cost-effective (if more resource-intensive) way of significantly reducing the enormous burden of depression that exists in developing regions.
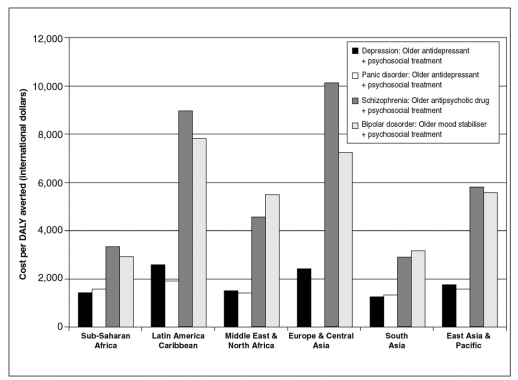
The considerable difference in cost-effectiveness between common and more severe mental disorders, as well as between low- and middle-income regions of the world, is clearly shown in Figure 1, which illustrates the ratios of cost to effect for a selective package of mental health interventions (one efficient treatment per disorder). Results for this baseline package indicate that, across six low- and middle-income regions, the potential total health gain emanating from such a combination of intervention strategies is in the order of 1,600-2,300 DALYs averted per one million total population, which could be achieved at an estimated cost of close to I$ 3-4 per capita in lowincome settings such as Sub-Saharan Africa and South Asia, and up to I$ 10 in middle-income regions (Latin America and the Caribbean; Europe and Central Asia). Two-thirds to three-quarters of the total costs of this package, but only about one third of the health gains are attributable to the more severe psychiatric conditions (schizophrenia and bipolar disorder). Approximately 300-500 healthy years of life can be gained for every investment of one million international dollars. Numerous other specifications are of course possible, including estimation of the costs and effects of a package that makes use of newer psychotropic drugs, or does not include any psychosocial treatment. Such comparisons reveal, for example, that substituting older with newer psychotropic drugs for the baseline package described above is anticipated to increase costs by 100-200% (an extra cost of I$ 4-7 per capita), while health gains would increase by 23-32%.
Figure 1.
Cost-effectiveness ratios for a basic mental health package in low- and middle-income regions of the world
DISCUSSION
This research report has set out the methods and results for the application of sectoral cost-effectiveness analysis to a range of psychiatric disorders that together represent an appreciable source of global disease burden. The purpose of such an analytical exercise is to locate the relative position of effective interventions within a wider cost-effectiveness framework in the health care sector. Using the criteria of the Commission for Macroeconomics and Health (15), the results of this analysis indicate that a) the most efficient interventions for common mental disorders (depression and panic disorder) can be considered very cost-effective (each DALY averted costs less than one year of average per capita income), and b) community-based interventions for more severe mental disorders using older antipsychotic and mood stabilising drugs meet the criterion for being cost-effective (each DALY averted costs less than three times the average annual income). These findings therefore provide relevant new information to health policy makers regarding the relative value of investing in psychiatric treatment and prevention, and in so doing may help to remove one of many remaining barriers to a more appropriate public health response to the burden of these conditions.
Importantly, however, the existence of such information at the highly aggregate level of WHO or World Bank regions is no guarantee that findings and recommendations will actually change health policy or practice at the national level (where policies are determined and resources actually allocated). Accordingly, there is a clear need to attempt a contextualisation of regional estimates down to this level, since many factors may alter the actual cost-effectiveness of a given intervention across settings, including the underlying epidemiology of disorders; the potential level of effective coverage in the population; the availability, mix and quality of inputs, especially personnel, drugs and consumables; and local prices, especially labour costs. Such a process of contextualisation has now been undertaken in a number of developing and developed countries in different WHO regions (including Estonia, Mexico, Nigeria, Sri Lanka and Spain), involving a step-by-step review and revision of regional model parameter values down to the local level (16). The output is a revised, population-specific set of average and incremental cost-effectiveness ratios for interventions addressing leading contributors to national disease burden.
Determination of the most cost-effective interventions for a particular mental disorder or condition, while informative in its own right, is not the end of the analytical process. Rather, it represents a key input into the broader task of priority-setting. For this task, the purpose is to go beyond efficiency concerns only. Other allocative criteria against which cost-effectiveness arguments need to be considered include the relative severity and the extent of spillover effects among different diseases, the potential for reducing catastrophic household spending on health, and protection of human rights. Thus, priority-setting necessarily implies a degree of trading-off between different objectives of the mental health system, such that the most equitable allocation of resources is highly unlikely to be the most efficient allocation. Within the mental health arena, schizophrenia treatment is an obvious example. While on pure efficiency grounds it could be overlooked in favour of cheaper and more cost-effective care and prevention strategies for more common mental disorders, this disorder is still typically included as a priority condition because of its severity (and consequent vulnerability of affected persons), its often catastrophic effect on the welfare and/or income of families and the socially valuable impact of treatment on individual-level symptoms and functioning. In addition, the relative merits of national or social insurance over private insurance and out-of-pocket expenditures as equitable mechanisms for safeguarding atrisk populations from the adverse financial consequences of mental disorders needs to be taken into account at the national level, as do the respective roles of public, private, voluntary and informal providers (17,18).
APPENDIX
WHO-CHOICE (CHOosing Interventions that are Cost-Effective) forms part of the work of the Department of Health System Financing, Expenditure and Resource Allocation at the World Health Organization in Geneva. The following colleagues have actively contributed to the conceptual and methodological development of WHO-CHOICE and are warmly acknowledged: Dr. Taghreed Adam, Dr. Rob Baltussen, Dr. David Evans, Raymond Hutubessy, Ben Johns, Jeremy Lauer, Dr. Christopher Murray and Dr. Tessa Tan Torres. In applying WHO-CHOICE to neuropsychiatric disorders, the following colleagues in the Department of Mental Health and Substance Abuse are particularly acknowledged for their contribution to data synthesis and interpretation: Dr. Mark van Ommeren (bipolar disorder and panic disorder) and Dr. Shekhar Saxena (schizophrenia and depression). Finally, since much of the foregoing analysis takes as its starting point the most recent Global Burden of Disease estimates for neuropsychiatric disorders, the underlying contribution of Dr. Jose-Luis Ayuso-Mateos and Dr. Bedirhan Ustun to this work is acknowledged. Needless to say, the views expressed rest with the author and are not necessarily those of the organization he serves.
References
- 1.World Health Organization. The world health report 2001. Mental health: new understanding, new hope. Geneva: World Health Organization; 2001. [Google Scholar]
- 2.Ustun B. Ayuso-Mateos JL. Chatterji S, et al. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:427–432. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 3.Singh B. Hawthorne G. Vos T. The role of economic evaluation in mental health care. Aust N Zeal J Psychiatry. 2001;35:104–117. doi: 10.1046/j.1440-1614.2001.00845.x. [DOI] [PubMed] [Google Scholar]
- 4.Shah A. Jenkins R. Mental health economic studies from developing countries reviewed in the context of those from developed countries. Acta Psychiatr Scand. 1999;100:1–18. doi: 10.1034/j.1600-0447.2000.9r004.x. [DOI] [PubMed] [Google Scholar]
- 5.Knapp MRJ. Almond S. Percudani M. Costs of schizophrenia: a review. In: Maj M, editor; Sartorius N, editor. Schizophrenia. Chichester: Wiley; 1999. pp. 407–454. [Google Scholar]
- 6.Rosenbaum JF. Hylan T. Costs of depressive disorders: a review. In: Maj M, editor; Sartorius N, editor. Depressive disorders. Chichester: Wiley; 1999. pp. 401–449. [Google Scholar]
- 7.Tan Torres T. Baltussen RM. Adam T, et al. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 8.Chisholm D. Sanderson K. Ayuso-Mateos JL, et al. Reducing the burden of depression: a population-level analysis of intervention cost-effectiveness in 14 epidemiologically-defined sub-regions (WHO-CHOICE) Br J Psychiatry. 2004;184:393–403. doi: 10.1192/bjp.184.5.393. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm D. Van Ommeren M. Ayuso-Mateos JL, et al. Costeffectiveness of clinical interventions for reducing the global burden of bipolar disorder: a global analysis (WHO-CHOICE) Br J Psychiatry. doi: 10.1192/bjp.187.6.559. in press. [DOI] [PubMed] [Google Scholar]
- 10.Hyman S. Chisholm D. Kessler R, et al. Mental disorders. In: Jamison D, editor; Breman J, editor; Measham A, et al., editors. Disease control priorities in developing countries. 2nd ed. New York: Oxford University Press; in press. [Google Scholar]
- 11.Ferri C. Chisholm D. Van Ommeren M, et al. Resource utilisation for neuropsychiatric disorders in developing countries: a multinational Delphi consensus study. Soc Psychiatry Psychiatr Epidemiol. 2004;39:218–227. doi: 10.1007/s00127-004-0729-5. [DOI] [PubMed] [Google Scholar]
- 12.Adam T. Evans D. Murray CJ. Econometric estimation of countryspecific hospital costs. Cost Effectiveness and Resource Allocation. 2003;(1):3. doi: 10.1186/1478-7547-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leucht S. Pitschel-Walz G. Abraham D, et al. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- 14.Mojtabai R. Nicolson RA. Carpenter BN. Role of psychosocial treatments in management of schizophrenia: a meta-analytic review of controlled outcome studies. Schizophr Bull. 1998;24:569–587. doi: 10.1093/oxfordjournals.schbul.a033350. [DOI] [PubMed] [Google Scholar]
- 15.Commission on Macroeconomics and Health. Macroeconomics and health: investing in health for economic development. Geneva: World Health Organization; 2001. [Google Scholar]
- 16.Hutubessy R. Chisholm D. Tan Torres T. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Effectiveness and Resource Allocation. 2003:1–8. doi: 10.1186/1478-7547-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine. Neurological, psychiatric and developmental disorders: meeting the challenge in the developing world. Washington: National Academy Press; 2001. [PubMed] [Google Scholar]
- 18.Jenkins R. McCulloch A. Friedli L, et al. Developing a national mental health policy. Maudsley Monograph 43. Hove: Psychology Press; 2002. [Google Scholar]