Abstract
PURPOSE: This pilot study examined osteopontin expression in uveal melanoma and focused on the role of measuring serum osteopontin to detect metastatic uveal melanoma.
METHODS: Osteopontin mRNA was measured in 3 uveal melanoma cell lines of varying invasive potential by real time PCR.Tissue sections of primary and metastatic uveal melanomas were stained for osteopontin. Serum osteopontin levels were measured by ELISA assays in 15 patients with metastatic uveal melanoma and in 37 patients who were disease-free for at least 10 years after treatment of the primary tumor. Paired serum samples drawn from 8 patients before and after development of metastasis were analyzed.
RESULTS: By real time PCR, highly invasive primary and metastatic uveal melanoma cells expressed 6 and 250 fold excess osteopontin mRNA respectively compared with poorly invasive primary uveal melanoma cells. Tissue sections of primary uveal melanomas lacking looping vasculogenic mimicry patterns either did not stain for osteopontin or exhibited weak diffuse staining. In primary melanomas containing looping vasculogenic mimicry patterns, strong osteopontin staining was detected in the tumor periphery where patterns were located. Diffuse strong expression of osteopontin was detected in 8 samples of metastatic uveal melanomas to the liver. Serum osteopontin levels were significantly higher in patients with metastatic uveal melanoma compared with patients who were disease-free for at least 10 years after treatment (p=0.0001) or with age matched controls. Serum osteopontin levels were significantly higher (p=0.008) after metastasis than before the detection of metastasis in 8 patients. When a cut-off value of 10ng/ml was used, the sensitivity and specificity of serum osteopontin in detecting metastatic melanoma was 87.5% and the area under the receiver operator characteristic curve was 96%.
CONCLUSIONS: Osteopontin is expressed diffusely in tissue sections of hepatic metastases from uveal melanoma and increased serum osteopontin levels correlate with metastatic melanoma to the liver with high specificity and sensitivity.
Uveal melanoma tends to spread first and preferentially to the liver.1 Currently, there is no effective treatment for metastatic uveal melanoma, in part because the metastatic tumor burden is typically high when liver metastases are detected by abnormalities in liver function tests or imaging studies.2-4 Our laboratory4 recently reported that the sensitivity and specificity of each of seven different liver function tests in detecting hepatic metastases from uveal melanoma was unsatisfactory for clinical use. Therefore, we continue to develop sensitive and specific biomarkers to detect metastatic uveal melanoma.
Osteopontin is a 314 amino acid phosphoglycoprotein that is a component of the non-collagenous bone matrix.5 Osteopontin has been described in the context of diverse physiological roles such as chemotaxis, cell migration, cell adhesion, angiogenesis, apoptosis, cell-extracellular matrix interactions, immune regulation and tumor metastasis.6
Osteopontin actively promotes the tumorigenic phenotype and contributes to metastasis. Increased osteopontin expression is associated with aggressive behavior and metastasis in breast, colon, prostate, lung, liver and ovarian cancers.7 Osteopontin is secreted into the blood where it can be detected by ELISA assays. Elevated serum osteopontin levels in the blood have been observed in patients with advanced or metastatic cancers.8-14 Recently, osteopontin expression has been shown to increase the invasive behavior of cutaneous melanoma cells.15 In this pilot study, we investigated the expression of osteopontin in uveal melanoma cell lines, in tissue sections of primary and metastatic uveal melanomas, and in the serum of patients treated for uveal melanoma as a potentially useful biomarker that correlates with metastasis to the liver.
MATERIALS AND METHODS
Cell lines
Cell Lines were derived from primary and metastatic uveal melanomas (M619 and MUM2B) as described previously.16 The OCM1a cell line17 was a generous gift from Dr. June Kan-Mitchell (Karmanos Cancer Institute, Wayne State University, Detroit, MI).These cell lines have been shown repeatedly to model the behavior of primary and metastatic uveal melanoma in vivo.16,18-21 The primary uveal OCM1a melanoma cell line is poorly invasive in membrane invasion culture system (MICS)22 assays and does not form vasculogenic mimicry patterns in three-dimensional cultures. By contrast, the M619 primary uveal melanoma cell line and the metastatic MUM2B uveal melanoma cell lines are highly invasive in MICS assays and form vasculogenic mimicry patterns in three-dimensional cultures.16 Melanoma cells were plated in DMEM (BioWhitaker, Inc., Walkersville, MD), and supplemented with 10% fetal bovine serum (Fisher, Ontario, Canada) without the addition of exogenous extracellular matrix molecules.
Real time PCR quantization of osteopontin mRNA
Osteopontin mRNA was quantified in the three uveal melanoma cell lines with different tumorigenic phenotypes. Total RNA was extracted using the RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Valencia, CA). Osteopontin mRNA was quantified by one step RTPCR in a Bio-Rad icycler iQ instrument using the Quantitect SYBR Green RT-PCR kit (Qiagen) using 2ng RNA as template. The following primers were used: Forward 5′ TGG CCG AGG TGA TAG TGT G 3′; Reverse 5′ CGG GGA TGG CCT TGT ATG 3′. Each 50μl reaction contained 1X buffer with dNTPs, RT enzyme, HotStarTaq DNA polymerase, 2.5mM MgCl2, 20pmols of each primer and 10nM fluorescein dye. Reverse transcription was at 50° for 45 minutes followed by 95° for 13.5 minutes to inactivate the RT enzyme and activate the HotStarTaq DNA polymerase. Subsequently, 42 cycles of PCR were performed: each cycle consisted of 95° for 20 seconds and 59° for 35 seconds. Fluorescence data was acquired during the combined annealing — extension phase of PCR. Finally a melt curve analysis was performed after PCR to confirm specificity. A standard curve was constructed using five 10-fold serial dilutions of in-vitro transcribed osteopontin RNA (2 × 106 copies to 2 × 102 copies). All standards and samples were run as two replicates.
Demonstration of osteopontin in histologic sections of primary and metastatic uveal melanomas
The detection of vasculogenic mimicry patterns in histological sections has been associated in multiple independent studies with death from metastatic uveal melanoma.23-27 Looping vasculogenic mimicry patterns include arcs, arcs with branching, loops and networks, and each of these patterns is associated with metastatic behavior.28 The parallel with cross-linking pattern is also associated with metastatic behavior.29 Vasculogenic mimicry patterns are associated with the presence of epithelioid cells29 and with monosomy 3,30 another highly reliable marker of metastatic behavior in uveal melanoma. Recently, an almost perfect association was shown between a gene expression profile that identified primary uveal melanomas at risk for metastasis and the presence of vasculogenic mimicry patterns.31 We therefore used the absence and presence of vasculogenic mimicry patterns as a histological marker for aggressive behavior in primary uveal melanomas.
We stained formalin fixed, paraffin-embedded tissue sections of four primary uveal melanomas that lacked any of the vasculogenic mimicry patterns associated with metastasis, 13 primary uveal melanomas that contained vasculogenic mimicry patterns, and 8 hepatic metastases from uveal melanoma for osteopontin. Tissue sections were hydrated to distilled water and were then placed in phosphate buffered saline (PBS) for 15 minutes followed by enzyme blocking for 10 minutes using peroxidase (Dako Peroxidase block; Dakocytomation, Carpinteria, CA) according to the manufacturer's instructions. Slides were rinsed with buffer then treated with protein block for 10 minutes. Tissue sections were blotted and incubated with a polyclonal antibody against osteopontin (ab8448; 1:400, Abcam Inc, Cambridge, MA) for 30 minutes, rinsed, and treated with EnVision Plus labeled polymer (Dakocytomation) for 30 minutes at room temperature. After sections were rinsed in buffer, the reaction product was detected by DAB Plus (Dako) for 10 minutes. Slides were rinsed in distilled water, counterstained, rinsed again in distilled water, dehydrated through an alcohol gradient, and mounted with Permount. In negative controls, primary antibody was replaced by buffer.
Serum Samples
Fifty two serum samples were obtained from the Ocular Oncology Serum Bank at Hadassah-Hebrew University Medical Center: 1 sample from each of 15 patients with metastatic uveal melanoma, 37 patients who were disease-free for at last 10 years following treatment of the primary tumor, and 30 age and sex-matched controls. Among the 15 patients with metastatic melanoma, there were 8 samples for which paired pre-metastatic sera were additionally available. Blood (7 cc) was drawn from patients at the time of diagnosis of the primary tumor before treatment and at least every six months after treatment. After collection, blood was centrifuged for 10 minutes at 1200 rpm. Serum was collected after centrifugation and was stored at -20°C.
Assay for Serum Osteopontin Levels
Serum levels of osteopontin were evaluated with an enzyme linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, MN) following the manufacturer's instructions. The kit is a 4.5 hour solid-phase ELISA designed to measure human osteopontin in serum and plasma. Results obtained using natural human osteopontin showed linear curves that were parallel to the standard curves obtained using the kit standards. These results indicated that the ELISA kit could be used to determine relative mass values for naturally occurring osteopontin.
The ELISA kit used employs a quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for osteopontin has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any osteopontin present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for OPN is added to the wells and color develops in proportion to the amount of osteopontin bound in the initial step. The color development is stopped and the intensity of the color is measured using a microplate reader at 450 nm. Results are calculated according to the standard curve created by the standards of osteopontin.
Human Subjects
The use of human tissue samples in these studies was approved by the Institutional Review Board of the University of Illinois at Chicago. The use of patient's sera was approved by the Hadassah-Hebrew University Medical Center Helsinki Committee.
RESULTS
Osteopontin expression in uveal melanoma cell lines:
Osteopontin is secreted and may bind to tumor cells. Therefore, to determine if melanoma cells are capable of generating osteopontin, we compared the expression of osteopontin mRNA in three uveal melanoma cell lines of varying invasive and metastatic behavior. Real time PCR quantitation showed that the highly invasive and metastatic uveal melanoma cell lines (M619, MUM2B) expressed 6 and 250 fold excess of osteopontin mRNA respectively compared with OCM1a cells.
Osteopontin expression in primary and metastatic uveal melanomas tissue sections
In 3 of 4 primary uveal melanomas lacking any of the vasculogenic mimicry patterns of prognostic significance, there was no evidence of staining for osteopontin; in 1 of these cases, diffuse weak cytoplasmic staining was noted. In 8 of 13 cases (62%) of primary uveal melanomas containing looping vasculogenic mimicry patterns associated with metastatic behavior, cytoplasmic staining was intense, confined to the periphery of the tumors, and tended to highlight looping patterns. In 5 of these 13 primary uveal melanoma cases, cytoplasmic staining for osteopontin was diffuse and weaker. Because macrophages are known to secrete osteopontin32,33 and because macrophages tend to be distributed in primary uveal melanomas within looping vasculogenic mimicry patterns,34 sections adjacent to tumors in which osteopontin decorated looping patterns were stained for the macrophage marker CD68. CD68-positive cells were detected within some areas of the patterns, but did not match the uniform distribution of osteopontin in these areas.Tissue sections of each of 8 deposits of metastatic uveal melanoma to the liver obtained from different patients all showed diffuse staining (cytoplasmic and nuclear) for osteopontin (Figure 1).
FIGURE 1.
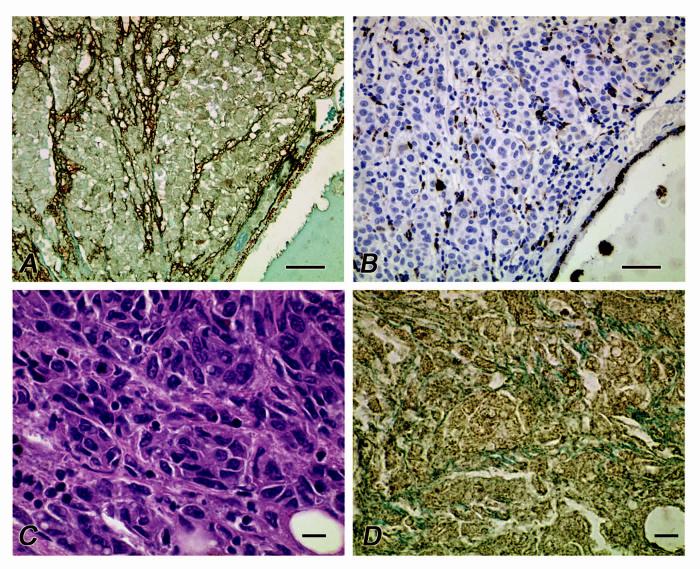
Distribution of osteopontin in primary and metastatic uveal melanoma. A. Osteopontin is distributed along looping vasculogenic mimicry patterns at the periphery of a primary uveal melanoma. Note the cytoplasmic staining of tumor cells and the absence of nuclear staining. B. Section adjacent to that illustrated in A., stained for CD68, a macrophage marker. Although macrophages are identified within vasculogenic mimicry patterns, the distribution of CD68-positive cells does not correspond to the diffuse staining of patterns seen when the tissue is labeled for osteopontin (A). C. Metastatic uveal melanoma to the liver. D. Section adjacent to C stained for osteopontin. Note the diffuse distribution of osteopontin in contrast with a primary uveal melanoma (A) and the presence of both nuclear and cytoplasmic staining. A, osteopontin counterstained with light green; B, CD68 counterstained with hematoxylin; C, hematoxylin-eosin, D. osteopontin counterstained with light green. Magnification bars: A, B = 50 μm; C,D = 10 μm.
Serum osteopontin levels
Serum osteopontin levels in patients with metastatic uveal melanoma were first compared with levels from patients who were disease free for at least 10 years after treatment of the primary uveal melanoma. The mean serum osteopontin level in patients with metastatic uveal melanoma was 17.62 ng/ml (n=15, S.E. 3.59, S.D. ± 13.89), where as the mean level in uveal melanoma patients who were disease free for at least 10 years was 7.15 ng/ml (n=37, S.E. = 0.49, S.D. ± 2.96; Kruskal-Wallis chi-square = 21.1296, df=1, p = 0.0001). Serum osteopontin levels were measured at least 6 months before the development of metastases in 8 of the 15 patients in this study that developed metastatic uveal melanoma to the liver. In these 8 patients, the mean pre-metastatic serum level of osteopontin was 6.19 ng/ml (S.E. = 1.05, S.D. ± 2.69) and the mean level of osteopontin at the time of the detection of metastasis was 19.66 (S.E. = 6.39, S.D. ± 18.07; sign test, p=0.008).
Serum levels of osteopontin from a group of 30 age- and sex-matched normal patients were compared with serum osteopontin levels from patients with metastatic uveal melanoma to the liver and to patients who were disease-free for at least 10 years. When controlling for age and sex, the serum level of osteopontin in patients with metastatic uveal melanoma to the liver was significantly higher than serum levels of normal controls (F=15.33, df=1, p=0.0004). There was no significant difference between serum osteopontin levels in the control group versus the group of patients who had survived disease free for at least 10 years after treatment of the primary tumor (F=0.23, df=1, p=0.6321), however, there was a significant difference between serum osteopontin levels in the group of long-term survivors and those patients who developed metastatic uveal melanoma to the liver when controlled for age and sex (F=24.94, df=1, p<0.0001). There were no significant differences in age (contingency chi-square = 3.6140, df=6, P = 0.7287) or distribution of gender (contingency chi-square = 1.0520, df=2, P = 0.5910) between the three populations studied (normal controls, long-term survivors, and patients with metastatic uveal melanomato the liver, compared by 3-way analysis of variance). By controlling for age and sex, the least square means of serum osteopontin levels were calculated: control patients = 6.74, long-term surviving patients =6.89, and patients with uveal metastatic melanoma = 18.25. These data are summarized in Table 1.
Table 1.
Comparison of Serum Osteopontin Levels by Group
| Control |
Metastatic Melanoma |
Long Term Survivors |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mean | sd | se | n | mean | sd | se | n | mean | sd | se | |
| Age | ||||||||||||
| <50 | 6 | 5.42 | 4.47 | 1.82 | 4 | 9.93 | 2.86 | 1.43 | 9 | 6.09 | 2.01 | 0.67 |
| 50-59 | 10 | 4.82 | 3.41 | 1.08 | 6 | 15.97 | 7.68 | 3.13 | 11 | 6.43 | 2.33 | 0.70 |
| 60-69 | 8 | 11.50 | 10.26 | 3.62 | 2 | 19.00 | 5.66 | 4.00 | 5 | 6.42 | 2.40 | 1.07 |
| ≥70 | 6 | 4.77 | 4.65 | 1.90 | 3 | 30.33 | 28.30 | 16.34 | 12 | 7.55 | 2.97 | 0.86 |
| Sex | ||||||||||||
| Male | 15 | 8.74 | 8.23 | 2.13 | 7 | 14.71 | 3.95 | 1.49 | 14 | 6.90 | 3.19 | 0.85 |
| Female | 15 | 4.68 | 3.95 | 1.02 | 8 | 20.19 | 18.86 | 6.67 | 23 | 6.59 | 1.99 | 0.41 |
| Overall | 30 | 6.71 | 6.70 | 1.22 | 15 | 17.63 | 13.88 | 3.58 | 37 | 6.70 | 2.47 | 0.41 |
With a cut-off value of 10ng/ml, the specificity of serum osteopontin for detecting metastasis was 83.7% in patients who remained disease free for more than 10 years. With the same cut-off value, both the specificity and sensitivity were 87.5% to detect metastasis in the patients with paired pre and postmetastasis samples. Receiver operator characteristic (ROC) curve analysis showed that the area under the curve was 96% with a cut-off value of 10ng/ml when pre- and post-metastatic osteopontin levels were analyzed in the paired samples.
DISCUSSION
In this study, we used a progressive series of experiments to investigate the potential role of serum osteopontin in the detection of metastatic uveal melanoma. We first detected an increase in the expression of osteopontin mRNA in aggressive primary M619 uveal melanoma cells relative to nonaggressive primary OCM1a uveal melanoma cells, and an even greater expression of mRNA in the metastatic MUM2B uveal melanoma cell line compared with the two primary melanoma cell lines. These data are important for two reasons. First, it is known that macrophages synthesize osteopontin,32,33 and the infiltration of macrophages into primary uveal melanomas has been associated with increased metastatic behavior in primary tumors.34 The high expression of osteopontin mRNA by metastatic uveal melanoma cells compared with aggressive and non-aggressive uveal melanoma cells suggested that the tumor cell itself may be a source of serum osteopontin. Second, the progressive increase in mRNA between highly invasive primary uveal melanoma cells (a 6X increase over non-invasive primary uveal melanoma cells) and metastatic uveal melanoma cells (a 250X increase over non-invasive primary uveal melanoma cells) suggested that we should prioritize the study of patient serum samples to the use of serum osteopontin to detect hepatic metastases. It may be worthwhile in a future study to investigate the role of osteopontin in stratifying patients with primary uveal melanocytic lesions into risk categories for metastasis using this simple blood test because a six fold change in mRNA expression in highly invasive melanoma cells compared with poorly invasive cells is significant.
We next confirmed differential osteopontin mRNA expression in cell lines by detecting osteopontin in tissue sections of primary uveal melanomas (with and without vasculogenic mimicry patterns) and in metastatic uveal melanoma by immunohistochemistry. In hepatic metastases from uveal melanoma, osteopontin expression was diffuse and strong (Figures 1C and 1D). In primary uveal melanomas, strong staining for osteopontin was confined to vasculogenic mimicry patterns at the periphery of the tumor (Figure 1A). This is consistent both with the tendency for these patterns to be localized at the tumor periphery35 and the tendency for osteopontin to be expressed at the peripheral tumor-stromal interface in highly malignant neoplasms.36 Cytoplasmic staining for osteopontin confirmed the observation from mRNA studies from cultured cells suggesting that melanoma cells generated osteopontin. CD68-positive cells (macrophages) were detected within these patterns as reported previously,37 but the distribution of these cells was scattered (Figure 1B), indicating that although tumor infiltrating macrophages may contribute to elevations in serum osteopontin, the greater contribution to serum osteopontin levels is most likely from melanoma cells.
It is also of interest that in histological sections of primary uveal melanomas lacking vasculogenic mimicry patterns, osteopontin was either not detected anywhere in the tumor or was expressed as weak diffuse cytoplasmic staining. These data suggest that it may be worthwhile to design a study to investigate the sensitivity and specificity of serum osteopontin levels in discriminating primary uveal melanomas lesions at low and high risk for metastasis.
This study focused on the potential use of serum osteopontin levels to detect metastatic uveal melanoma. Consistent with observations that blood levels of osteopontin are useful markers of metastatic behavior in a variety of cancers,8-14 we observed that patients with metastatic uveal melanoma had significantly higher levels of serum osteopontin when compared with patients who remained disease free for at least 10 years. Both sensitivity and specificity of serum osteopontin in detecting metastasis in uveal melanoma patients after treatment of the primary lesion is 87.5%, and analysis of the area under the ROC curve indicates that the probability of a correct diagnosis of metastasis based on a serum osteopontin level is 96%. Recent data from the authors' own laboratories4 indicate that the sensitivity and specificity of six liver function tests including bilirubin, gamma glutamyl transpeptidase (γGT), alkaline phosphates (ALK), lactate dehydrogenase (LDH), aspartate-aminotrasferase (AST), and alanine-aminotrasferase (ALT) is low compared with the sensitivity and specificity of serum osteopontin to detect hepatic metastases reported in this study. For example, at the time of the diagnosis of hepatic metastasis, the sensitivity of ALT was 40%, the sensitivity for AST and γGT was 50%, and the sensitivity of ALK and bilirubin was 60%. The sensitivity of LDH was 80% at the time of the detection of hepatic metastasis, but elevated LDH is not specific for hepatic metastasis. Data on liver function tests from our laboratory are consistent with reports from other investigators who discovered that the sensitivity of γGT is 21%, the sensitivity of ALK is 25%,38 and the sensitivity of LDH is 67%.3,39 Thus, based on this pilot study, serum osteopontin levels appear to be both more specific and sensitive than any currently available hepatic function test.
It is especially significant that the serum level of osteopontin appears to increase as metastases develop, consistent with the positive expression of osteopontin in tissue sections of metastatic uveal melanoma to the liver (Figure 1). Thus, it may be useful to obtain blood to establish the serum level of osteopontin before treatment of the primary lesion, and to follow these serum levels sequentially. Elevation of serum osteopontin over a period of time, analogous to the monitoring elevations in blood prostatic specific antigen to identify patients at risk for metastatic prostatic carcinoma,40,41 may be useful in identifying early metastasis from uveal melanoma.
In this study, the association between elevated serum osteopontin levels in patients with early metastatic uveal melanoma is consistent with the increased osteopontin expression by different types of highly invasive and metastatic tumor cells.8-14 By contrast, an increased expression of osteopontin mRNA from primary uveal melanoma tissues was associated with improved rather than decreased survival in two gene-expression array studies of primary uveal melanoma tissue.42,43 Because osteopontin is expressed preferentially in the periphery of primary uveal melanomas, the inhomogeneous distribution of tumor cells expressing osteopontin may result in a systematic sampling bias if tissue for gene expression studies is extracted from the center of the tumor.
Additional studies are required on larger patient samples to validate the results of this pilot study. Also, it may be useful to include serum osteopontin levels in a panel of serum biomarkers including serum melanoma inhibitory activity (MIA).44 MIA, secreted by the melanoma cell line HTZ-19,45 was detected in histological sections of primary uveal melanoma and metastatic uveal melanoma to the liver by immunohistochemistry. Additionally, serum levels of MIA detected by enzyme-linked immunosorbent assay (ELISA) increased significantly when patients developed metastases to the liver, thus demonstrating the feasibility of utilizing serum levels of molecules overexpressed in melanoma to detect metastatic uveal melanoma.44By using a panel of serum biomarkers, including osteopontin, it may be possible to achieve an outstanding sensitivity and specificity in detecting early metastasis from uveal melanoma. Discovery of early metastasis while the hepatic tumor burden is relatively small may be helpful in designing strategies to treat patients at high risk of dying from metastatic uveal melanoma.
Footnotes
Supported by National Eye Institute Grant EY10457.
Reference List
- McLean IW. The biology of haematogenous metastasis in human uveal malignant melanoma. Virchows Arch A Pathol Anat. 1993;422:433–437. doi: 10.1007/BF01606450. [DOI] [PubMed] [Google Scholar]
- Donoso LA, Shields JA, Augsburger JA, Orth DH, Johnson P. Metastatic uveal melanoma: diffuse hepatic metastasis in a patient with concurrent normal serum enzyme levels and liver scan. Arch Ophthalmol. 1985;103:758. doi: 10.1001/archopht.1985.01050060016002. [DOI] [PubMed] [Google Scholar]
- Eskelin S, Pyrhonen S, Summanen P, Prause JU, Kivela T. Screening for metastatic malignant melanoma of the uvea revisited. Cancer. 1999;85:1151–1159. [PubMed] [Google Scholar]
- Kaiserman I, Amer R, Pe'er J. Liver function tests in metastatic uveal melanoma. Am J Ophthalmol. 2004;137:236–243. doi: 10.1016/j.ajo.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–184. [PubMed] [Google Scholar]
- Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal H, Bautista DS, Tonkin KS, et al. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res. 1997;3:605–611. [PubMed] [Google Scholar]
- Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060–4066. [PubMed] [Google Scholar]
- Tuck AB, Chambers AF. The role of osteopontin in breast cancer: clinical and experimental studies. J Mammary Gland Biol Neoplasia. 2001;6:419–429. doi: 10.1023/a:1014734930781. [DOI] [PubMed] [Google Scholar]
- Kim JH, Skates SJ, Uede T, et al. Osteopontin as a potential diagnostic biomarker for ovarian cancer. JAMA. 2002;287:1671–1679. doi: 10.1001/jama.287.13.1671. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Mima T, Ishii T, et al. Enhanced production of osteopontin in multiple myeloma: clinical and pathogenic implications. Br J Haematol. 2003;123:263–270. doi: 10.1046/j.1365-2141.2003.04589.x. [DOI] [PubMed] [Google Scholar]
- Koopmann J, Fedarko NS, Jain A, et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487–491. [PubMed] [Google Scholar]
- Martinetti A, Bajetta E, Ferrari L, et al. Osteoprotegerin and osteopontin serum values in postmenopausal advanced breast cancer patients treated with anastrozole. Endocr Relat Cancer. 2004;11:771–779. doi: 10.1677/erc.1.00775. [DOI] [PubMed] [Google Scholar]
- Zhou YW, Dai DL, Martinka M, et al. Osteopontin expression correlates with melanoma invasion. J Invest Dermatol. 2005;124:1044–1052. doi: 10.1111/j.0022-202X.2005.23680.x. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan-Mitchell J, Mitchell MS, Rao N, Liggett PE. Characterization of uveal melanoma cell lines that grow as xenografts in rabbit eyes. Invest Ophthalmol Vis Sci. 1989;30:829–834. [PubMed] [Google Scholar]
- Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Kirschmann DA, et al. Molecular determinants of uveal melanoma metastasis. Clin Exp Met. 2002;19:233–246. doi: 10.1023/a:1015591624171. [DOI] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–3075. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Karavitis J, Valyi-Nagy K, et al. Chromatin sensitivity to Alu I endonuclease is regulated by extracellular matrix and the cytoskeleton. Am J Pathol. 2005;166:1187–1203. doi: 10.1016/S0002-9440(10)62338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Fidler IJ. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987;38:137–147. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakamoto M, Yoshikawa H, et al. Histologic findings and prognosis of uveal malignant melanoma in Japanese patients. Am J Ophthalmol. 1996;121:276–283. doi: 10.1016/s0002-9394(14)70275-2. [DOI] [PubMed] [Google Scholar]
- Schaling DF, van der Pol JP, Schlingemann RO, Parys-Van Ginderdeuren R, Jager MJ. Vascular density and vascular patterns in the prognosis of choroidal melanoma. In: Schaling DF, editor. Radionucleotides and Radiolabelled Antibodies in Choroidal Melanoma (Diagnosis and Therapy) Rijksuniversiteit te Leiden; Leiden: 1996. pp. 43–54. [Google Scholar]
- McLean IW, Keefe KS, Burnier MN. Uveal melanoma: comparison of the prognostic value of fibrovascular loops, mean of the ten largest nucleoli, cell type and tumor size. Ophthalmology. 1997;104:777–780. doi: 10.1016/s0161-6420(97)30234-6. [DOI] [PubMed] [Google Scholar]
- Seregard S, Spangberg B, Juul C, Oskarsson M. Prognostic accuacy of the mean of the largest nucleoli, vascular patterns, and PC-10 in posterior uveal melanoma. Ophthalmology. 1998;105:485–491. doi: 10.1016/S0161-6420(98)93032-9. [DOI] [PubMed] [Google Scholar]
- Makitie T, Summanen P, Tarkannen A, Kivela T. Microvascular loops and networks as prognostic indicators in choroidal and ciliary body melanomas. J Nat Cancer Inst. 1999;91:359–367. doi: 10.1093/jnci/91.4.359. [DOI] [PubMed] [Google Scholar]
- Folberg R, Chen X, Boldt HC, et al. Microcirculation patterns other than loops and networks in choroidal and ciliary body melanomas. Ophthalmology. 2000;108:996–1001. doi: 10.1016/s0161-6420(01)00541-3. [DOI] [PubMed] [Google Scholar]
- Folberg R, Rummelt V, Parys-Van Ginderdeuren R, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100:1389–1398. doi: 10.1016/s0161-6420(93)31470-3. [DOI] [PubMed] [Google Scholar]
- Scholes AGM, Damato BE, Nunn J, et al. Monosomy 3 in uveal melanoma: Correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44:1008–1011. doi: 10.1167/iovs.02-0159. [DOI] [PubMed] [Google Scholar]
- Onken MD, Lin AY, Worley LA, Folberg R, Harbour JW. Association between microarray gene expression signature and extravascular matrix patterns in uveal melanoma. Am J Ophthalmol. 2005 doi: 10.1016/j.ajo.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Brown LF, Papadopoulos-Sergiou A, Berse B, et al. Osteopontin expression and distribution in human carcinomas. Am J Pathol. 1994;145:610–623. [PMC free article] [PubMed] [Google Scholar]
- Bourassa B, Monaghan S, Rittling SR. Impaired anti-tumor cytotoxicity of macrophages from osteopontin-deficient mice. Cell Immunol. 2004;227:1–11. doi: 10.1016/j.cellimm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Makitie T, Summanen P, Tarkkanen A, Kivela T. Tumor-infiltrating macrophages (CD68(+) cells) and. prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421. [PubMed] [Google Scholar]
- Folberg R, Fleck M, Mehaffey MG, et al. Mapping prognostically significant vascular patterns in ciliary body and choroidal melanomas. Pathol Oncol Res. 1996;2:229–236. doi: 10.1007/BF02904815. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathol Int. 2002;52:19–24. doi: 10.1046/j.1440-1827.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- Makitie T, Summanen P, Tarkkanen A, Kivela T. Tumor infiltrating macrophages, microvessels, and prognosis in malignant melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421. [PubMed] [Google Scholar]
- Hicks C, Foss AJ, Hungerford JL. Predictive power of screening tests for metastasis in uveal melanoma. Eye. 1998;12(Pt 6):945–948. doi: 10.1038/eye.1998.245. [DOI] [PubMed] [Google Scholar]
- Char DH. Metastatic choroidal melanoma. Am J Ophthalmol. 1978;86:76–80. doi: 10.1016/0002-9394(78)90018-1. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- Walsh PC. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. J Urol. 2005;173:830. doi: 10.1097/01.ju.0000151994.12709.12. [DOI] [PubMed] [Google Scholar]
- Tschentscher F, Husing J, Holter T, et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res. 2003;63:2578–2584. [PubMed] [Google Scholar]
- Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller UC, Bosserhoff AK, Neubauer AS, et al. Melanoma inhibitory activity: a novel serum marker for uveal melanoma. Melanoma Res. 2002;12:593–599. doi: 10.1097/01.cmr.0000043146.28051.b8. [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK, Kaufmann M, Kaluza B, et al. Melanoma-inhibiting activity, a novel serum marker for progression of malignant melanoma. Cancer Res. 1997;57:3149–3153. [PubMed] [Google Scholar]


