Abstract
Hypoglycemia-induced Counterregulatory failure is a dangerous complication of insulin use in diabetes mellitus. Controlled hypoglycemia studies in gene knockout models, which require the use of mice, would aid in identifying causes of defective counterregulation. Because stress can influence Counterregulatory hormones and glucose homeostasis, we developed glucose clamps with remote blood sampling in conscious, unrestrained mice. Male C57BL/6 mice implanted with indwelling carotid artery and jugular vein catheters were subjected to 2 h of hyperinsulinemic glucose clamps 24 h apart, with a 6-h fast before each clamp. On day 1,, blood glucose was maintained (euglycemia, 178 ± 4 mg/dl) or decreased to 62 ± 1 mg/dl (hypoglycemia) by insulin (20 mU·kg−1·min−1) and variable glucose infusion. Donor blood was continuously infused to replace blood sample volume. Baseline plasma epinephrine (32 ± 8 pg/ml), corticosterone (16.1 ± 1.8 μg/dl), and glucagon (35 ± 3 pg/ml) were unchanged during euglycemia but increased significantly during hypoglycemia, with a glycemic threshold of ~80 mg/dl. On day 2, all mice underwent a hypoglycemic clamp (blood glucose, 64 ± 1 mg/dl). Compared with mice that were euglycemic on day 1, previously hypoglycemic mice had significantly higher glucose requirements and significantly lower plasma glucagon and corticosterone (n = 6/group) on day 2. Epinephrine tended to decrease, although not significantly, in repeatedly hypoglycemic mice. Pre- and post-clamp insulin levels were similar between groups. We conclude that counterregulatory responses to acute and repeated hypoglycemia in unrestrained, chronically cannulated mice reproduce aspects of counterregulation in humans, and that repeated hypoglycemia in mice is a useful model of counterregulatory failure.
Keywords: hypoglycemia-associated autonomic failure, hypoglycemia unawareness, catecholamines, norepinephrine, glucocorticoids
hypoglycemia-associated counterregulatory failure is an increasingly common complication of intensive insulin therapy and poses new obstacles to glucose control in insulin-dependent diabetes, Counterregulatory failure is evident as decreases in either the absolute level or the threshold for activation of sympathoadrenal, glucocorticoid, and glucagon responses to hypoglycemia (3). Of these responses, the glucagon and sympathoadrenal responses are the most critical because of the rapidity with which they are activated and serve to increase blood glucose (4). Counterregulatory failure is not unique to individuals with type 1 diabetes and can be demonstrated experimentally in nondiabetic subjects after only one to two episodes of hypoglycemia (5, 6). However, because type 1 diabetes patients lack the ability to decrease insulin and often lose hypoglycemia-induced glucagon secretion with increased duration of their disease (3), they are in danger of the central nervous system effects of severe hypoglycemia, including loss of consciousness, coma, or even death, when sympathoadrenal responses are impaired by recurrent hypoglycemia. Similar risk factors have also been demonstrated for late-stage type 2 diabetics who require insulin therapy (3).
Identifying the factors that contribute to counterregulatory failure will aid in reducing the potentially fatal risks of insulin therapy. Several mechanisms have been proposed, including increased brain glucose uptake (1), elevations in nonglucose cerebral energy substrates (13, 44), hypoglycemia-induced release of glucocorticoids (7), corticotropin-releasing hormone (CRH), or CRH-related peptides (17) and inhibition of hind-brain noradrenergic neurons (36). However, support for these mechanisms has not been documented consistently (3, 11), and the causes of counterregulatory failure are likely to be multi-factorial.
Elucidation of counterregulatory mechanisms would be aided by well-controlled studies of hypoglycemia in gene knockout models, which require the use of mice. Glucose clamps in mice commonly involve either acute vascular catheterization surgery and anesthesia (34) or tail nick sampling and restraint (12, 16, 30). However, surgery, pain, handling, and restraint are all stresses that can activate sympathoadrenal and adrenocortical activity (24, 33). Stress and autonomic activation can also stimulate glucagon secretion (42, 45). The potential impact of elevations in these counterregulatory hormones is supported by experimental evidence in humans and animals that stress can induce hyperglycemia (41). The balance between catecholamines, glucocorticoids, and glucagon can have distinct effects on glycogenolysis, gluconeogenesis, and glucose flux across specific tissues (18, 21), such that, depending on its nature and duration, stress could confound the characterization of metabolic as well as hormonal aspects of counterregulation. Therefore, glucose clamp techniques that minimize potential confounding effects of stress on counterregulatory responses are needed in mice. We have developed techniques for glucose clamps and remote blood sampling in chronically cannulated, conscious, unrestrained C57BL/6 mice. With these techniques, we have tested the effects of acute and repeated hypoglycemia on counterregulation in the mouse.
METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committees of Albany Medical College and Vanderbilt University. Male C57BL/6 mice (JAX Research Systems, Bar Harbor, ME) were housed on a 12:12-h light-dark cycle (lights on at 6 AM) and used at 2 mo of age (24 ± 0.4 g body wt). Mice were implanted with chronic indwelling carotid artery and jugular vein catheters under pentobarbital sodium anesthesia (50 mg/kg, ip), as previously described (22). The catheters were tunneled under the skin to exit in the suprascapular area. Catheters were flushed daily with heparin (200 U/ml) after surgery. Mice were used for glucose clamp studies 5–7 days after surgery, when they had regained their preoperative body weight.
Glucose clamps
On the day of the study, mice were transferred within 1 h of lights-on to 1-liter chambers with bedding but no food or water. At this time, extension lines were attached to the arterial and venous catheters and exteriorized through a lid on the chamber. Mice were studied in the postabsorptive state starting 7 h after lights-on. After a baseline arterial blood sample (160 μl), regular insulin (Humulin-R; Lilly, Indianapolis, IN) was infused intravenously at 20 mU·kg−1·min−1 for 120 min, combined with a variable infusion of glucose to match the baseline incoming blood glucose for each mouse (euglycemia) or to maintain target blood glucose levels of 70 mg/dl (hypoglycemia). In preliminary experiments, we determined that the insulin infusion rate was necessary to decrease glucose consistently and maintain target levels for ≥60 min, the length of hypoglycemia indicated by our previous work to encompass peak and plateau counterregulatory hormone responses (28). Glucose was measured every 10 min in whole arterial blood (5 μl) with a glucose meter (Hemocue, Lake Forest, CA). Larger arterial samples (160 μl) were drawn for analysis of plasma hormones at 30 and 120 min. To replace volume and red cells lost in sampling, heparinized blood cells from donor mice were resuspended in saline and infused intravenously, along with the insulin and glucose, at 6 μl/min.
Once the 120-min blood sample had been collected on day 1, insulin infusion was stopped and glucose infusion was continued to restore euglycemia in hypoglycemic mice. Mice were then returned to their home cages with food and water overnight. To assess effects of repeated hypoglycemia on counterregulatory responses, all mice were subjected on the next day (day 2) to a hyperinsulinemic hypoglycemic glucose clamp. All day 2 procedures were the same as they were for the day 1 hypoglycemic clamp, except that an additional 160-μl blood sample was collected at 15 min for blood glucose and plasma hormones. Mice were killed immediately after the 120-min blood sample on day 2 by pentobarbital sodium overdose (100 mg/kg iv) and decapitation.
Plasma assays
Plasma epinephrine and norepinephrine (lower detection limit, 20 pg/ml) were measured in 50-μl plasma samples by HPLC according to previously described methods (20). The inter-assay coefficient of variation for epinephrine was 10% for the low control (~30 pg/ml) and 3.4% for the high control (~500 pg/ml). The interassay coefficient of variation for norepinephrine was 8.5% for the low control (~300 pg/ml) and 4.0% for the high control (~800 pg/ml). Plasma corticosterone [MP Biomedical (formerly ICN), Irvine, CA] and insulin (Linco, St. Louis, MO) were measured in 2.5- and 25-μl samples, respectively, using radioimmunoassays previously validated in mice for these volumes (2, 26, 28). Glucagon was assayed in 20-μl plasma samples, using a radioimmunoassay from Linco; the assay had a sensitivity of 10 pg/ml and interassay coefficients of variation of 8.6% for samples in the 55–70 pg/ml range and 8.2% for samples in the 215–250 pg/ml range (2, 26, 28).
Statistics
Data were analyzed by two-way ANOVA with repeated measures across day and time. Post hoc tests comparing two groups of mice at individual time points were performed by t-test with Bonferroni correction (Statview 5.0; SAS Institute, Cary, NC). Significance was defined as P < 0.05. Data are presented throughout as means ± SE.
RESULTS
Two groups of mice were studied. One group was exposed to hyperinsulinemic hypoglycemia on both day 1 and day 2 (Hypo-Hypo). The second group was exposed to a hyperinsulinemic euglycemic clamp on day 1 and a hypoglycemic hyperinsulinemic clamp on day 2 (Eu-Hypo). Blood glucose levels for day 1 of the sequential clamps are shown in Table 1. Baseline blood glucose was similar between groups and averaged 173 ± 6 mg/dl for all mice (n = 12). Average glucose levels on day 1 during the last hour of the clamp in Hypo-Hypo mice were 62 ± 1 (n =6). Average glucose levels in Eu-Hypo mice during the last hour (178 ± 4 mg/dl; n = 6/group) were comparable to initial blood glucose levels. Glucose requirements in Hypo-Hypo mice on day 1 increased to a plateau of 15 ± 1 mg·kg−1·min−1 during the last hour of study. The glucose infusion required to maintain euglycemia was higher at all times (Table 1) and was 79 ± 2 mg·kg−1·min−1 during the last hour of the clamp on day 1 (n = 6/group).
Table 1.
Day 1 blood glucose and GIR in mice, subjected to Eu-Hypo or Hypo-Hypo hyperinsulinemic glucose clamps
| Time, min | 0 | 10 | 15 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose, mg/dl | Eu-Hypo | 183±9 | 232+16 | 173+11 | 154±9 | 157±7 | 156±8 | 167±8 | 184±6 | 191±11 | 184±9 | 184+7 | 182±9 | 169+12 | 149±13 |
| Hypo-Hypo | 162±8 | 94±6 | 83±6 | 85±4 | 82±4 | 79±3 | 74±4 | 68±2 | 64±2 | 60±3 | 61+2 | 61 ±4 | 62±2 | 61±2 | |
| GIR, mg·kg−1·min−1 | Eu-Hypo | 56±1 | 54±2 | 60±3 | 70±3 | 75 ±4 | 82±5 | 84±4 | 83 ±5 | 79±4 | 79±4 | 78±5 | 76±5 | 78±3 | 78±3 |
| Hypo-Hypo | 0 | 0 | 0 | 0.7±.7 | 4±2 | 4±1 | 6±2 | 9±2 | 12±1 | 15±2 | 15±2 | 17+2 | 19+3 | 19±3 |
Values are means ± SE; n = 6 per group. GIR, glucose infusion rate; Eu-Hypo, mice subjected to a euglycemic clamp on day 1 followed by a hypoglycemic clamp on day 2; Hypo-Hypo, mice exposed to a hypoglycemic clamp on days 1 and 2.
Baseline levels of epinephrine were low and near the limit of detection (pooled 0-min levels for all mice on day 1, 32 ± 8 pg/ml; n = 12). Plasma norepinephrine was <300 pg/ml in all but two mice (pooled 0-min levels for all mice on day 1, 185 ± 33 pg/ml; n = 12). Plasma glucagon was also near the lower detection limit in most samples (pooled 0-min levels for all mice on day 1, 35 ± 3 pg/ml; n = 12). Initial plasma corticosterone levels on day 1 were 16.1 ± 1.8 μg/dl (n = 12).
Only mice subjected to hypoglycemia exhibited increases in counterregulatory hormones on day 1 (Fig. 1). Plasma epinephrine, corticosterone and glucagon increased in Hypo-Hypo but not in Eu-Hypo mice on day 1. Plasma norepinephrine did not change in either group (Fig. 1). We estimated the threshold for epinephrine, corticosterone, and glucagon responses to hypoglycemia by determining the glucose level at which each hormone exceeded the 95% confidence limit (39) of levels measured in euglycemic mice. Plasma epinephrine, corticosterone, and glucagon were consistently increased at glucose levels in the 70–80 mg/dl range (Fig. 2).
Fig. 1.
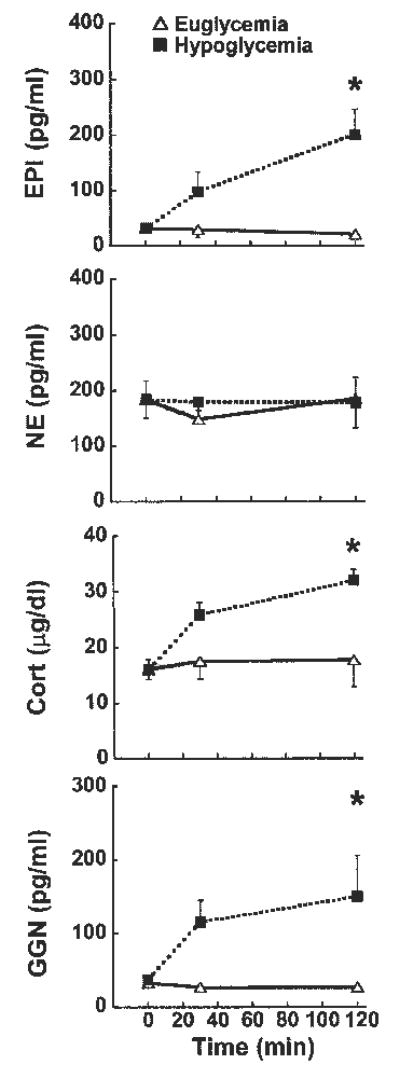
Plasma levels of epinephrine (EPI; top), norepinephrine (NE; upper middle), corticosterone (Cort; lower middle), and glucagon (GGN; bottom) in mice exposed to a hypoglycemic (▪) or a euglycemic (▵) clamp on day 1. ANOVA main effects are summarized in Table 3; n = 6/group. *P < 0.05, Eu-Hypo vs. Hypo-Hypo at the same time point.
Fig. 2.
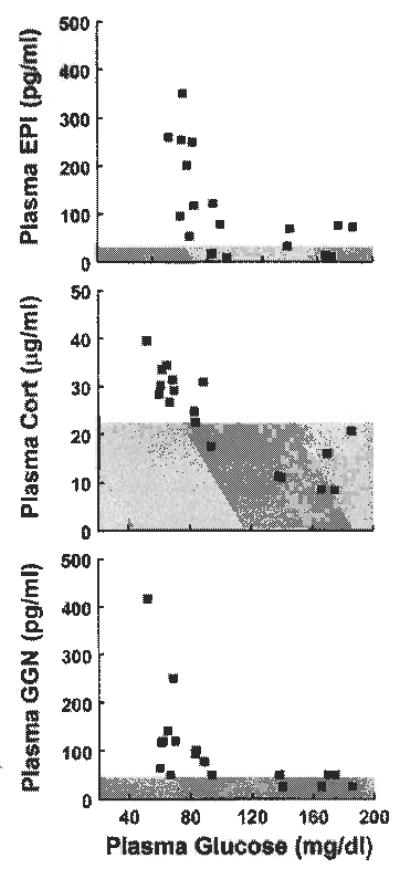
Plasma EPI (top), Cort (middle), and GGN (bottom) vs. blood glucose levels in individual Hypo-Hypo mice on day 1. Shaded area indicates upper 95% confidence limit for levels of each hormone measured in Eu-Hypo mice on day 1. Plasma NE is not shown because it did not change significantly during hypoglycemia; n = 6/group
On day 2, baseline blood glucose levels were similar between Eu-Hypo and Hypo-Hypo groups (Fig. 3, bottom). Glucose levels were also similar between Eu-Hypo and Hypo-Hypo mice at all times during insulin-induced hypoglycemia on day 2, such that neither the absolute levels nor the rate of fall differed between groups (Fig. 3, bottom). The average glucose levels during the last hour of hypoglycemia on day 2 were 65 ± 1 and 63 ± 1 mg/dl in Hypo-Hypo and Eu-Hypo mice, respectively. A significantly higher rate of glucose infusion was required to maintain equivalent glucose levels in Hypo-Hypo vs. Eu-Hypo mice during the first 70 min of hypoglycemia on day 2 (Fig. 3, top). Total glucose requirements, calculated as the area under the glucose infusion rate curve, were also significantly higher in Hypo-Hypo mice (Hypo-Hypo vs. Eu-Hypo, 2,531 ± 374 vs. 1,161 ± 207 mg/kg, n = 6). On both study days, plasma insulin levels were similar at baseline and increased to comparable levels after each clamp (Table 2).
Fig. 3.
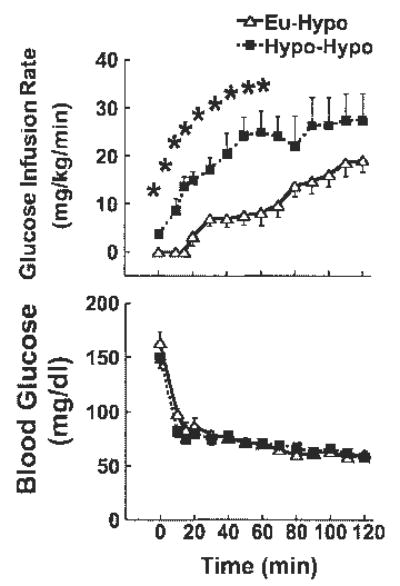
Glucose infusion rates (top) and blood glucose levels (bottom) during hypoglycemia in mice previously exposed to either hypoglycemia (Hypo-Hypo; ▪) or euglycemia (Eu-Hypo; ▵) 1 day earlier. Insulin was infused for 120 min at 20 mU·kg−1·min−1 starting at time 0. ANOVA main effects are summarized in Table 3. Blood glucose did not differ between Hypo-Hypo and Eu-Hypo mice at any time by post hoc testing despite significant main effects by ANOVA; n = 6/group. *P < 0.05, Eu-Hypo vs. Hypo-Hypo at the same time point.
Table 2.
Baseline (0 min) and final (120 min) plasma insulin levels produced by 120-min hyperinsulinemic glucose clamps in Eu-Hypo and Hypo-Hypo mice
|
Day 1 |
Day 2 |
|||
|---|---|---|---|---|
| 0 min | 120 min | 0 min | 120 min | |
| Eu-Hypo | 1.9±0.8 | 15.2±2.7 | 1.4 ±0.2 | 16.4 ± 2.6 |
| Hypo-Hypo | 1.0±0.2 | 15.9±1.6 | 0.65 ±0.1 | 13.7 ± 1.8 |
Values are means ± SE in ng/ml and are as described in methods; n = 6/group.
Baseline hormone levels on day 2 were not affected by prior exposure to hypoglycemia or euglycemia on day 1 (Fig. 4). Plasma epinephrine tended to be lower on day 2 in Hypo-Hypo vs. Eu-Hypo mice, but this effect was not significant (Fig. 4, top). Norepinephrine levels on day 2 also did not differ between Hypo-Hypo and Eu-Hypo mice. However, increases in plasma corticosterone on day 2 were significantly less by 120 min in Hypo-Hypo vs. Eu-Hypo mice (Fig. 4, lower middle). Plasma glucagon responses to hypoglycemia on day 2 were also blunted at 15 min in Hypo-Hypo mice (Fig. 4, bottom). Area under the plasma glucagon curve was also significantly reduced in Hypo-Hypo mice (Hypo-Hypo vs. Eu-Hypo, 426 ± 163 vs. 1,385 ± 116; n = 6/group). There were no significant differences in area under the curve for plasma epinephrine, corticosterone, or norepinephrine (not shown). Post hoc comparisons between day 1 and day 2 responses to hypoglycemia in Hypo-Hypo mice did show that 120-min epinephrine levels were lower after repeated hypoglycemia. However, after correcting post hoc test results for the comparison between Hypo-Hypo and Eu-Hypo mice, this decrease was not significant (day 1 vs. day 2, 203 ± 45 vs. 149 ± 41 pg/ml, n = 6/group; P = 0.048 before and 0.096 after correction). Comparison of other endpoints between day 1 and day 2 in Hypo-Hypo mice did not reveal any further effects of repeated hypoglycemia on counterregulatory hormones beyond those discussed above. More severe hypoglycemia (final hour average blood glucose, 42 ± 2 mg/dl; n = 4/group) increased day 1 peak catecholamine, corticosterone, and glucagon responses by ~1,400, 55, and 450%, respectively, but did not result in greater inhibition of plasma catecholamines or other counterregulatory hormones on day 2 (data not shown).
Fig. 4.
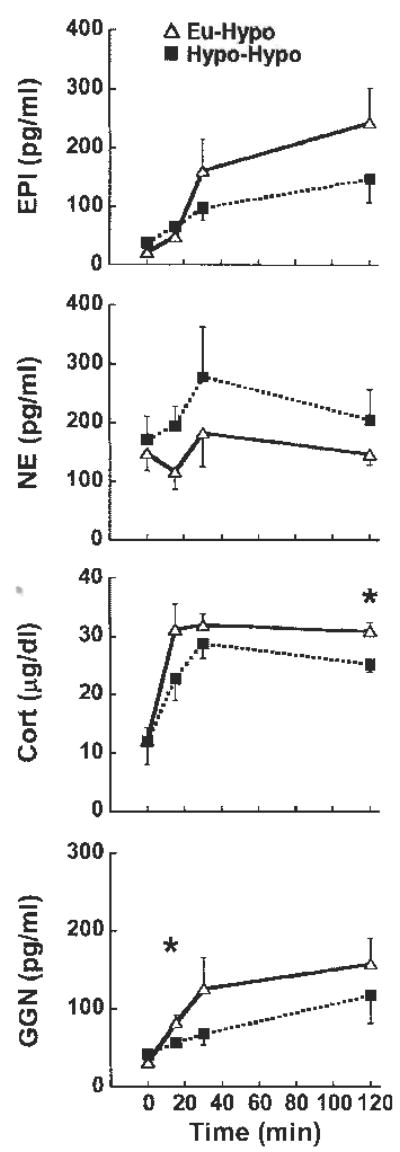
Plasma levels of EPI (top), NE (upper middle), Cort (lower middle), and GGN (bottom) during hypoglycemia in mice previously exposed to hypoglycemia (Hypo-Hypo; ▪) or euglycemia (Eu-Hypo; ▵) 1 day earlier. ANOVA main effects are summarized in Table 3; n = 6/group. *P < 0.05, Eu-Hypo vs. Hypo-Hypo at the same time point.
DISCUSSION
We have established a hypoglycemic glucose clamp model that permits sequential glucose clamps and repeated hormone sampling with minimal extraneous stress in mice. Using this model, we have demonstrated hypoglycemia-specific stimulation of epinephrine, glucocorticoid, and glucagon secretion, as well as defective counterregulation after repeated exposure to controlled hypoglycemia.
Baseline plasma epinephrine levels were typically low and near the limit of detection on both days of sequential glucose clamps. Although initial levels of plasma corticosterone in our studies seem elevated compared with circadian trough levels measured in fed mice (27, 32), this is probably an effect of fasting. Glucocorticoid levels in the present study were comparable to those measured at midday in similarly fasted, unoperated mice (L. Jacobson, unpublished observations). Baseline epinephrine and corticosterone levels were ~30- and 3-fold lower, respectively, than those reported in mice that were restrained and sampled from the tail tip (16). Neither plasma catecholamines nor glucocorticoids increased significantly during a euglycemic clamp combined with repeated blood sampling. Our model therefore minimizes stress-associated effects on counterregulatory hormones and metabolic parameters.
As has been shown in other species (5, 15, 39, 40, 47), clamping glucose at hypoglycemic but not euglycemic levels significantly increased plasma epinephrine, corticosterone, and glucagon in male C57BL/6 mice. Counterregulatory hormone responses were also related to stimulus intensity, with deeper hypoglycemia eliciting higher hormone levels. Although the frequency and total number of hormone samples in our experiments were insufficient to derive a precise glycemic threshold, correlation of hormone levels against blood glucose indicated that epinephrine, corticosterone, and glucagon increased in mice at glucose levels that were close to 80 mg/dl. This level is notably higher than the 60–70 mg/dl threshold reported for responses of these counterregulatory hormones to insulin- induced hypoglycemia in humans and dogs (25, 39) and suggests that the same blood glucose level may not represent the same stimulus severity in all species. We did not observe hypoglycemia-induced increases in plasma norepinephrine, but elevated norepinephrine is not a consistent feature of counterregulation in other species (14, 29, 37–39). Thus counterregulatory hormone responses to controlled, acute hypoglycemia in the mouse are largely comparable to those in other species.
Mice also resemble other species in exhibiting evidence of impaired glucose regulation after limited antecedent exposure to hypoglycemia. Studies in nondiabetic humans indicate that as little as a single or two brief (10–30 min) prior episodes of hypoglycemia are sufficient to diminish counterregulatory hormone and endogenous glucose production responses to subsequent hypoglycemia (5, 6). We observed counterregulatory deficits in mice after a single 2-h episode of hypoglycemia, the most dramatic being the marked increase in glucose infusion requirements, which are likely to reflect decreased endogenous glucose production (4–6, 23, 46). Comparable to the blunting of counterregulatory hormones described in humans (7, 23) and rats (14, 35, 38, 40), mice also had significantly lower glucocorticoid and glucagon responses after exposure to repeated hypoglycemia. The reduced glucagon response might have accounted in part for the increased glucose requirements during the first hour of day 2 hypoglycemia (4, 9), although significant increases in glucose infusion rates before 15 min suggest that glucagon or other counterregulatory factors could have been reduced at even earlier times in Hypo-Hypo mice.
We did not observe significant hypoglycemia-associated inhibition of epinephrine when responses were compared between previously euglycemic and previously hypoglycemic mice. However, we did detect decreases in epinephrine responses between the first and second exposures to hypoglycemia within the Hypo-Hypo group, suggesting that repeated hypoglycemia had the expected inhibitory effects on sympathoadrenal activity, even if those effects did not reach statistical significance in multiple comparisons. It is unlikely that hypoglycemia was insufficiently prolonged or severe enough to impair sympathoadrenal responsiveness. Mice were hypoglycemic for as long as the time shown to reduce hypoglycemia-induced plasma catecholamines in humans (6, 8), and antecedent hypoglycemia of ~40 mg/dl did not further inhibit day 2 epinephrine responses. Conversely, it also seems unlikely that hypoglycemia was too severe. Because epinephrine and glucagon responses did not exceed 300 pg/ml, responses to even milder hypoglycemia might have been too low to detect decrements due to repeated hypoglycemia. We doubt that limited group size prevented us from detecting significant differences between Hypo-Hypo and Eu-Hypo epinephrine responses. In subsequent studies of gene knockout models, we have found that wild-type controls on a C57BL/6 background exhibit counterregulatory hormone responses to repeated hypoglycemia that are statistically indistinguishable from those reported here. Pooling data from these and the present experiments, representing a total of 16 Hypo-Hypo mice and 11 Eu-Hypo mice, did not alter results from those described above. Comparisons between Eu-Hypo and Hypo-Hypo mice still showed significant reductions after repeated hypoglycemia only in glucagon and corticosterone and not in epinephrine (28a).
The lack of more robust inhibition of epinephrine responses by recurrent hypoglycemia could indicate that counterregulatory mechanisms in mice differ from those in humans and other species. Nevertheless, many studies (5, 6, 8, 17, 38,40,46) that demonstrate evidence of counterregulatory deficits do not show decreases in all counterregulatory hormones at once. Even some human studies (5) do not find decreases in epinephrine when other counterregulatory hormones are decreased. Instead, we suspect that inconsistencies between the effects of repeated hypoglycemia on sympathoadrenal responses in mice and humans are due to blood sampling constraints in mice. Increased sampling frequency, made possible by larger blood volume in humans, increases the chances of detecting changes in absolute, incremental, or integrated hormonal responses. Because even significant hormonal differences on day 2 between Hypo-Hypo and Eu-Hypo mice were relatively transient, our limited hormone sampling schedule may have precluded the detection of more significant sympathoadrenal inhibition.
It is possible that factors we did not measure were more significantly affected by repeated hypoglycemia in mice. We did not analyze growth hormone, because it was reported to have a minor role in counterregulation in mice (43). However, this conclusion might not apply to recurrent hypoglycemia in the mouse. We also did not have any measurements of sympathetic activity other than plasma norepinephrine and epinephrine. Because plasma norepinephrine might not be a reliable indicator of sympathetic nerve activity, particularly during hypoglycemia (10, 19), we may have missed changes in sympathetic outflow induced by repeated hypoglycemia. Changes in lactate or ketone production, which we did not assess, have also been suggested to contribute to the suppression of counterregulatory responses after recurrent hypoglycemia (44). However, because counterregulatory impairment can occur independently of changes in lactate or ketones (6, 13), these factors do not appear to be major mediators of hypoglycemia-induced decreases in counterregulatory hormones. These considerations notwithstanding, it is equally plausible that the decrements in epinephrine, corticosterone, and glucagon, regardless of whether they were statistically significant, had a cumulative impact to increase glucose requirements. Other mechanisms, including alterations in insulin secretion, endogenous glucose production, or tissue glucose uptake, can be readily investigated in our model.
In summary, we have developed a model for remote infusion and blood sampling to evaluate counterregulation with minimal stress in the conscious, unrestrained mouse. We have demonstrated that counterregulatory hormone responses and glucose requirements during acute and repeated hypoglycemia in mice resemble those in humans and other species. This model will be valuable for testing the role of individual genes in normal and defective counterregulation through the use of knockout and transgenic models.
Table 3.
| P Values | |||||||
|---|---|---|---|---|---|---|---|
| Glucose | GIR | EPI | NE | Cort | GGN | Insulin | |
| Group | < 0.0001 | < 0.0001 | 0.2656 | 0.3721 | 0.7270 | 0.2161 | 0.3902 |
| Day | < 0.0001 | < 0.0001 | 0.0783 | 0.8777 | 0.1823 | 0.1828 | 0.8401 |
| Group × day | < 0.0001 | < 0.0001 | 0.0427 | 0.3355 | 0.0290 | 0.0166 | 0.7957 |
| Time | < 0.0001 | < 0.0001 | 0.0015 | 0.8083 | < 0.0001 | 0.0005 | < 0.0001 |
| Group × time | < 0.0001 | 0.1997 | 0.877 | 0.2522 | 0.0994 | 0.5500 | 0.9381 |
| Day × time | < 0.0001 | 0.7175 | 0.0927 | 0.6793 | 0.0029 | 0.4272 | 0.9446 |
| Group × day × time | <0.0001 | < 0.0001 | 0.0151 | 0.7859 | 0.0019 | 0.0491 | 0.6948 |
EPI, epinephrine; NE, norepinephrine; Cort, corticosterone; GGN, glucagon. Group refers to whether mice were hypoglycemic or euglycemic on day 1. Day indicates day 1 vs. day 2 hyperinsulinemic glucose clamps. Time refers to time during a given glucose clamp. Significant values (P < 0.05) are highlighted in bold.
Acknowledgments
We are grateful to Rebecca Kittell (Albany Medical College), Carlo Malabanan, Wanda Snead, Jamie Yates, and Jessica Potts (Vanderbilt University Mouse Metabolic Phenotyping Center) for technical assistance and to Dr. David Wasserman (Vanderbilt University) for input into and enthusiasm for this project.
Footnotes
GRANTS
The research was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-62442 (L. Jacobson), DK-59757 (L. Jacobson), and DK-59637 (O. P. McGuinness).
References
- 1.Boyle PJ, Kempers SF, O'Connor AM, Nagy RJ. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Met. 1995;333:1726–1731. doi: 10.1056/NEJM199512283332602. [DOI] [PubMed] [Google Scholar]
- 2.Brissova M, Nicholson WE, Shiota M, and Powers AC. Assessment of insulin secretion in the mouse. In; Methods in Molecular Medicine, edited by Ozcan S. Totowa, NJ: Humana, 2003, p. 23–45. [DOI] [PubMed]
- 3.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 5.Davis MR, Shamoon H. Counterregulatory adaptation to hypoglycemia in normal humans. J Clin Endocrinol Metab. 1991;73:995. doi: 10.1210/jcem-73-5-995. [DOI] [PubMed] [Google Scholar]
- 6.Davis SN, Mann S, Galassetti P, Neill RA, Tate D, Ertl AC, Costa F. Effects of differing durations of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia in normal humans. Diabetes. 2000;49:1897–1903. doi: 10.2337/diabetes.49.11.1897. [DOI] [PubMed] [Google Scholar]
- 7.Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest. 1996;98:680–691. doi: 10.1172/JCI118839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis SN, Shavers C, Mosqueda-Garcia R, Costa F. Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes. 1997;46:1328–1335. doi: 10.2337/diab.46.8.1328. [DOI] [PubMed] [Google Scholar]
- 9.DeFeo P, Perriello G, Torlone E, Fanelli C, Ventura MM, Santeusanio F, Brunetti P, Gerich JE, Bolli GB. Evidence against important catecholamine compensation for absent glucagon counterregulation. Am J Physiol Endocrinol Metab. 1991;260:E203–E212. doi: 10.1152/ajpendo.1991.260.2.E203. [DOI] [PubMed] [Google Scholar]
- 10.DeRosa MA, Cryer PE. Hypoglycemia and the sympathoadrenal system: neurogenic symptoms are largely the result of sympathetic neural, rather than adrenomedullary, activation. Am J Physiol Endocrinol Metab. 2004;287:E32–E41. doi: 10.1152/ajpendo.00539.2003. [DOI] [PubMed] [Google Scholar]
- 11.DcVries MC, Lawson MA, Beverly JL. Dissociation of hypothalamic noradrenergic activity and sympathoadrenal responses to recurrent hypoglycemia. Am J Physiol Regul Integr Camp Physiol. 2004;286:R910–R915. doi: 10.1152/ajpregu.00254.2002. [DOI] [PubMed] [Google Scholar]
- 12.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lcpori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 13.Evans ML, Matyka K, Lomas J, Pernet A, Cranston IC, MacDonald I, Amiel SA. Reduced counterregulation during hypoglycemia with raised circulating nonglucose lipid substrates: evidence for regional differences in metabolic capacity in the human brain? J Clin Endocrinol Metab. 1998;83:2952–2959. doi: 10.1210/jcem.83.8.4937. [DOI] [PubMed] [Google Scholar]
- 14.Evans SB, Wilkinson CW, Bentson K, Gronbeck P, Zavosh A, Figlewicz DP. PVN activation is suppressed by repeated hypoglycemia but not antecedent corticosterone in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1426–R1436. doi: 10.1152/ajpregu.2001.281.5.R1426. [DOI] [PubMed] [Google Scholar]
- 15.Fanelli C, Pampanelli S, Epifano L, Ciofetta M, Modarelli F, Di Vincenzo A, Annibale B, Lepore M, Lalli C, DelSindaco P, Brunetti P, Bolli GB. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologica. 1994;37:797–807. doi: 10.1007/BF00404337. [DOI] [PubMed] [Google Scholar]
- 16.Fisher SJ, Brüning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes. 2005;54:1447–1451. doi: 10.2337/diabetes.54.5.1447. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan DE, Keshavarz T, Evans ML, Flanagan S, Fan X, Jacob RJ, Sherwin RS. Role of corticotropin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes. 2003;52:605–613. doi: 10.2337/diabetes.52.3.605. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara T, Cherrington AD, Neal DN, McGuinness OP. Role of cortisol in the metabolic response to stress hormone infusion in the conscious dog. Metabolism. 1996;45:571–578. doi: 10.1016/s0026-0495(96)90026-8. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS. Clinical assessment of sympathetic responses to stress. Ann NY Acad Sci. 1995;771:570–593. doi: 10.1111/j.1749-6632.1995.tb44711.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein DS, Feuerstein G, Izzo JJL, Kopin IJ, Keiser HR. Validity and reliability of liquid chromatography with electrochemical detection for measuring plasma levels of norepinephrine and epinephrine in man. Life Sci. 1981;28:467–475. doi: 10.1016/0024-3205(81)90139-9. [DOI] [PubMed] [Google Scholar]
- 21.Gustavson SM, Chu CA, Nishizawa M, Farmer B, Neal D, Yang Y, Vaughan S, Donahue EP, Flakoll P, Cherrington AD. Glucagon’s actions are modified by the combination of epinephrine and gluconeogenic precursor infusion. Am J Physiol Endocrinol Metab. 2003;285:E534–E544. doi: 10.1152/ajpendo.00059.2003. [DOI] [PubMed] [Google Scholar]
- 22.Halseth AE, Bracy DP, Wasserman DH. Overexpression of hexokinase II increases insulin- and exercise-stimulated muscle glucose uptake in vivo. Am J Physiol Endocrinol Metab. 1999;276:E70–E77. doi: 10.1152/ajpendo.1999.276.1.E70. [DOI] [PubMed] [Google Scholar]
- 23.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after one episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamopituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Igawa K, Mugavero M, Shiota M, Neal DW, Cherrington AD. Insulin selectively controls the glucagon response to mild hypoglycemia in the dog. Diabetes. 2002;51:3033–3042. doi: 10.2337/diabetes.51.10.3033. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson L. Glucocorticoid replacement, but not CRH deficiency, prevents adrenalectomy-induced anorexia in mice. Endocrinology. 1999;140:310–317. doi: 10.1210/endo.140.1.6416. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson L. Lower weight loss and food intake in protein-deprived, CRH-deficient mice correlates with glucocorticoid insufficiency. Endocrinology. 1999;140:3543–3551. doi: 10.1210/endo.140.8.6910. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson L, Pacak K. Counterregulatory responses after repeated hypoglycemia are not enhanced by combined corticotropin-releasing hormone and glucocorticoid deficiency in mice. Metabolism. 2005;54:1259–1265. doi: 10.1016/j.metabol.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Jacobson L, Ansari T, Potts J, and McGuinness OP. Glucocorticoid-deficient corticotropin-releasing hormone knockout mice maintain glucose requirements but not automatic responses during repeated hypoglycemia. Am J Physiol Endocrinol Metab [ePub Jan. 31, 2006] doi:10.1152/ajpendo.00523.2005. [DOI] [PMC free article] [PubMed]
- 29.Kerr D, MacDonald IA, Tattersall RB. Influence of duration of hypoglycemia on the hormonal counterregulatory response in normal subjects. J Clin Endocrinol Metab. 1989;68:1118–1122. doi: 10.1210/jcem-68-6-1118. [DOI] [PubMed] [Google Scholar]
- 30.Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim HJ, Yu C, Chen Y, Qu X, Haluzik M, Reitman ML, Shulman GI. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes. 2003;52:1311–1318. doi: 10.2337/diabetes.52.6.1311. [DOI] [PubMed] [Google Scholar]
- 32.Muglia LJ, Jacobson L, Weninger SC, Luedke CE, Bae DS, Jeong KH, Majzoub JA. Impaired diurnal adrenal rhythmicity restored by constant infusion of corticotropin-releasing hormone in CRH-deficient mice. J Clin Invest. 1997;99:2923–2929. doi: 10.1172/JCI119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1247–R1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 34.Pacini G, Thomaseth K, Ahren B. Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab. 2001;281:E693–E703. doi: 10.1152/ajpendo.2001.281.4.E693. [DOI] [PubMed] [Google Scholar]
- 35.Powell AM, Sherwin RS, Shulman GI. Impaired hormonal responses to hypoglycemia in spontaneously diabetic and recurrently hypoglycemic rats. J Clin Invest. 1993;92:2667–2674. doi: 10.1172/JCI116883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholaminergic subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–201. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- 37.Rossi G, Lapaczewski F, Diamond MP, Jacob RJ, Shulman GI, Sherwin RS. Inhibitory effect of pregnancy on counterregulatory hormone responses to hypoglycemia in awake rat. Diabetes. 1993;42:1440–1445. doi: 10.2337/diab.42.10.1440. [DOI] [PubMed] [Google Scholar]
- 38.Sandoval DA, Ping L, Neill AR, Morrey S, Davis SN. Cortisol acts through central mechanisms to blunt counterregulatory responses to hypoglycemia in conscious rats. Diabetes. 2003;52:2198–2204. doi: 10.2337/diabetes.52.9.2198. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. 1987;79:777–781. doi: 10.1172/JCI112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shum K, Inouye K, Chan O, Mathoo J, Bilinski D, Matthews SG, Vranic M. Effects of antecedent hypoglycemia, hyperinsulinemia, and excess corticosterone on hypoglycemic counterregulation. Am J Physiol Endocrinol Metab. 2001;281:E455–E465. doi: 10.1152/ajpendo.2001.281.3.E455. [DOI] [PubMed] [Google Scholar]
- 41.Surwit RS, Schneider MS, Feinglos MN. Stress and diabetes mellitus. Diabetes Care. 1992;15:1413–1422. doi: 10.2337/diacare.15.10.1413. [DOI] [PubMed] [Google Scholar]
- 42.Taborsky GJ, Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired α-cell responses in type 1 diabetes. Diabetes. 1998;47:995–1005. doi: 10.2337/diabetes.47.7.995. [DOI] [PubMed] [Google Scholar]
- 43.Tamaki M, Sato M, Niimi M, Takahara J. Resistance of growth hormone secretion to hypoglycemia in the mouse. J Neuroendocrinol. 1995;7:371–376. doi: 10.1111/j.1365-2826.1995.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 44.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Effect of hyperketonemia and hyperlacticacidemia on symptoms, cognitive dysfunction, and counterregulatory hormone responses during hypoglycemia in normal humans. Diabetes. 1994;43:1311–1317. doi: 10.2337/diab.43.11.1311. [DOI] [PubMed] [Google Scholar]
- 45.Watters JM and Wilmore DW. The metabolic responses to trauma and sepsis. In: Endocrinology (2nd ed.), edited by DeGroot LJ. Philadelphia, PA: Saunders, 1989, p. 2367–2393.
- 46.Widom B, Simonson DC. Intermittent hypoglycemia impairs glucose counterregulation. Diabetes. 1992;41:1597–1602. doi: 10.2337/diab.41.12.1597. [DOI] [PubMed] [Google Scholar]
- 47.Zinker BA, Allison RG, Lacy DB, Wasserman DH. Interaction of exercise, insulin, and hypoglycemia studied using euglycemic and hypoglycemic clamps. Am J Physiol Endocrinol Metab. 1997;272:E530–E542. doi: 10.1152/ajpendo.1997.272.4.E530. [DOI] [PubMed] [Google Scholar]


