Abstract
The body mass index (BMI) is often used as a predictor of overweight and obesity. There is, however, an important debate among international specialists as to what the risk limits should be, and where the cut-off points should be located. In the United States, for instance, adults with a BMI between 25 and 30 are considered overweight, while adults with a BMI of 30 or higher are considered obese. Nevertheless some researchers, especially in developing countries, claim that the limits established for the US are too permissive, and that the threshold to define obese adults should be set lower for other nationalities and ethnicities.
This paper analyzes the mortality risks for different BMI levels of two populations of American adult men. The first population lived during the last quarter of the 19th century and the early 20th century. These men were drawn from a random sample of Union Army veterans who fought during the American Civil War (1861–1865). A contemporary sample of men was drawn from the first wave of the National Health and Nutrition Examination Survey (NHANES I) conducted between 1971 and 1975. The results indicate that the frontier of overweight and obesity are expanding over time, such that the potential risk is nowadays associated with higher levels of BMI. The finding may imply that differences in BMI cut-off points are not only cross ethnic, but also occur for similar ethnicities across time.
Keywords: BMI, Obesity, Mortality, Health, Union Army, NHANES I
1. Introduction
Over the course of the 20th century, obesity has grown dramatically in the United States. During the last decade of the 19th century, around 3.5% of white middle aged men where obese. A century later, more than 35% of the comparable population was considered obese (Helmchen and Henderson, 2004).1 As Komlos and Baur (2004) point out, the American population went from being the tallest in the world during the 19th century to one of the heaviest at the end of the 20th century. Yet the obesity problem is not confined to the United States. International organizations such as the World Health Organization (WHO) and the International Obesity Task Force (IOTF) have recognized obesity as a worldwide epidemic (James et al., 2001). Even in developing countries obesity has become a public health problem, to the point that some households now share both undernutrition and obesity within its members (Popkin, 2002).2
One of the problems that researchers face when analyzing obesity prevalence and trends, is the comparability of measurements across populations. In order to measure overweight and obesity, the body mass index (BMI) is widely utilized as a predictor of the healthy levels of weight for height among populations and individuals. The BMI or Quetelet index relates height to weight (weight (kg)/height (m2)) and is commonly used as an indirect measure of adiposity in the human body. Different levels of BMI are associated with morbidity and mortality risk levels, and the level of body fat or adipose tissue stored in the body. The evidence shows that across populations in time and space both very low and very high levels of BMI are unhealthy and increase the probability of morbidity and mortality (Troiano et al., 1996; Waaler, 1984; Costa, 1993; Costa and Steckel, 1997; Fogel, 1993; Fogel, 1994; Visscher et al., 2000).
For more than a decade, analysts in the nutrition and anthropometrics fields have been debating whether a uniform set of BMI ranges can be applied to all populations and subpopulations across time, space and groups. For instance, most evidence points to a different physiology among contemporary Caucasians and Asians. Associated risks tend to appear at lower BMI ranges for Asians than for Caucasians. But this has not always been the case. In the United States, men born during the mid-19th century had higher risks at lower BMIs than contemporary Americans.
This paper compares BMI risk across populations by contrasting two cohorts of American men that were born almost a century apart. A 19th century cohort is represented by a group of Union Army veterans, whereas the 20th century cohort was drawn from the first wave of the National Health and Nutrition Examination Survey (NHANES I). I argue that the problems for comparing cut-off points for overweight and obesity are found not only across different racial and ethnic groups, but also across comparable populations over time. Nineteenth century adult men had a higher mortality risk at lower BMI levels than the contemporary cohort.
2. Overweight adults
When humans ingest more energy than they spend, the excess energy is stored in the form of adipose tissue. Energy storage is fundamental for survival. Whenever food is scarce, the body uses the stored fat to provide additional energy. As more fat is stored, resulting from an energy surplus, the risks of morbidity and mortality increase. In other words, “obesity may be defined as the degree of fat storage associated with clearly elevated health risks.” (WHO, 1995). Therefore for health purposes obesity is not an aesthetic consideration, but rather a risk factor. It represents excessive body mass for stature, and more specifically an excessive body fat content. The effects of obesity are debilitating both socially and physically (Bray et al., 1998).
There are several methods to measure obesity or excess body fat. Among the alternatives, BMI is often used as an indicator of obesity. While there are other alternatives to BMI that are more accurate in terms of measuring areas of regional fat, BMI is a good and reliable predictor of total fat, and it is relatively inexpensive to measure.3 BMI measurements facilitate the comparison between past and present populations and across countries, especially given that other measurements are not readily available for poor countries. Even in richer countries, like the US, other obesity tests remain too expensive and prevent the use of large samples. In addition, the use of BMI as a proxy for overweight and obesity is a relatively easy method for policy recommendations and communicating targets to the population at large.
Given the considerations mentioned, BMI is widely accepted as a predictor of obesity. The consensus, however, breaks down among the international community of researchers when establishing the boundaries that predict obesity and overweight. In the United States, for instance, adults with a BMI of 30 or more are considered to be obese (CDC, 2004), a limit that has been also established by the World Health Organization (WHO, 1998). Nevertheless, some researchers find a BMI of 30 too permissive for certain ethnic groups. In the year 2000, a panel of experts coordinated by the World Health Organization produced a report to assess the risks of different BMIs in Asian Populations (WHO, 2000). The report concluded that Asians in general had higher health risks than Caucasians for the same BMIs and therefore that the limits of overweight and obesity should be set lower for Asian adults. Popkin argues that there is limited but strong evidence that the biology among sub-populations is different in relation to the BMI risks, “[t]hat is, Asians, Africans, and Latin Americans are more likely than whites in the USA and Europe to have greater body fat and central fat for the same BMI and to have a higher likelihood of experiencing cardiovascular disease (CVD) outcomes of importance at lower BMI levels” (Popkin, 2002, p. 100).
Table 1 presents the recommendations of a number of selected international boards of experts. The table clearly shows that there are marked differences in the recommendations, not only internationally and among different study groups, but the same expert boards have established different limits at different times. In the United States, most boards agree on cut-off points of 25 and 30 kg/m2 for overweight and obesity respectively. The 1989 report of the National Research Council (NRC), however, considered a BMI of 27 as the threshold for overweight. In Australia and Canada the recommendations are similar to those in the United States. By contrast, Asians are considered overweight at 23 kg/m2 and obese at 25 kg/m2. In Mexico, adults of short stature (women below 1.5 m and men below 1.6 m) share the same cut-off points as Asians. As in the Mexican case, some experts have differentiated obesity risk limits by gender showing the importance of sex specific cutoff points. For example, during the mid 1980s, the National Institutes of Health (1985) concluded that men with a BMI over 27.8 and women with a BMI over 27.3 were obese.
Table 1.
Classification of desirable weight levels as assessed by the BMI according to selected expert panels
| BMI recommendations
|
|||||||
|---|---|---|---|---|---|---|---|
| Country/region | Panel | Gender | Group | Date | Normal | Overweight | Obese |
| Asia | IOTF/IASO/WHO (a) | Both | Adults | 2000 | 18.5–22.9 | 23–24.9 | ≥25 |
| Australia | NHMRC (b) | Both | Adults | 1997 | 25–30 | ≥30 | |
| Canada | Health and Welfare (c) | Both | Adults | 1988 | 20–25 | 25–27 | ≥27 |
| Canada | Health and Welfare (d) | Both | Adults | 2003 | 18.5–24.9 | 25–29.9 | ≥30 |
| Mexico | SSA (e) | Female | Adults with height ≥ 1.5 m | 1998 | 25–27 | ≥27 | |
| Female | Adults with height < 1.5 m | 23–25 | ≥25 | ||||
| Male | Adults with height ≥ 1.6 m | 25–27 | ≥27 | ||||
| Male | Adults with height < 1.6 m | 23–25 | ≥25 | ||||
| United States | NIH/NHLBI/NASSO (f) | Both | Adults | 2000 | 18.5–24.9 | 25–29.9 | ≥30 |
| United States | National Research Council (g) | Both | Ages 19–34 | 1989 | 19–25 | ||
| Ages 35+ | 21–27 | ||||||
| United States | USDA/DHHS (h) | Both | Adults | 2000 | 18.5–25 | 25–30 | ≥30 |
| United States | CDC (i) | Both | Adults | 2004 | 18.5–24.9 | 25–29.9 | ≥30 |
| World | WHO (j) | Both | Adults | 1998 | 18.5–24.9 | 25–29.9 | ≥30 |
Sources: (a) IOTF (2000); (b) NHMRC (1997); (c) Health and Welfare Canada (1988); (d) Health and Welfare Canada (2003); (e) Secretaría de Salubridad y Asistencia (1998); (f) NIH (2000); (g) NRC (1989); (h) USDA/USDHH (2000); (i) CDC (2004); and (j) WHO (1998).
To a large degree the recommendations of some panels respond to pragmatic considerations and convenience. While overweight and obesity increase the risk of morbidity and mortality, the risk of particular diseases may increase at different BMI levels. In order to simplify the use of cutoff points, Garrow (1981) suggested adopting a single set of divisions using 5 BMI unit intervals (Bray et al., 1998). The WHO and most expert committees in the United States have followed Garrow’s approach. The National Institutes of Health recommend to the general public the following guidelines4 (Table 2).
Table 2.
NIH recommendations for weight classification
| Type | BMI |
|---|---|
| Underweight | Below 18.5 (to 20 following Garrow) |
| Normal | 18.5–24.9 |
| Overweight | 25–29.9 |
| Obesity class I (high) | 30–34.9 |
| Obesity class II (very high) | 35–39.9 |
| Obesity class III (extremely high) | 40 and above |
Source: NIH, 2004.
Yet, not only overweight generates health problems, being underweight is also liked to poor health.5 In an influential 1984 study of a very large sample of Norwegians, Hans Waaler outlined the U-shaped relationship between BMI and morbidity and mortality, such that extreme BMIs are riskier than those at the center of the distribution. The graphical depiction of these relationships has been called “Waaler curves,” an analytical instrument that has served to indicate optimal anthropometrics from a health standpoint.6 Several follow-up studies have further supported Waaler’s findings and support the U-shape relationship between BMI and mortality for other populations in different regions and historical periods (Fogel, 1993; Kim, 1996; Allison and Faith, 1996; Allebeck and Bergh, 1992; Engeland et al., 2003; Song et al., 2003; Strandberg, 1997). The proposed weight-to-height minimum risks vary according to age, race, gender, disease history and behavioral factors such as smoking.
3. Data: The Union Army and NHANES I
The present study compares two samples of American adult men. The first sample was drawn from a group of Union Army (UA) veterans who fought during the American Civil War (1861–1865). Over the past two decades, Robert Fogel and his associates at the Center for Population Economics have compiled the life histories of around 40,000 UA volunteer white soldiers who were randomly chosen at the National Archives (Fogel, 1999, 2000a). The American Civil War marked a whole generation and a large number of northern men served in the Union Army. The sample is therefore representative of Northern white men of military age in the early 1860s (Fogel, 2001). Veterans who survived thewar were eligible for pensions, a program that became so large that by the early 20th century 90% of the survivors were on the pension rolls (Glasson, 1918).7 Pension applicants were examined by a board of surgeons who determined the degree of disability and the amount of money that should be awarded. During the medical examination, the height and weight of pension applicants were recorded. Hence, it is possible to calculate the BMI of the veterans at the time of the examination. For most recruits, the date of death was also recorded, so we can trace them from the time of the examination to their death, and some cases, the cause of death was documented.
The second sample of men was drawn from the first wave of National Health and Nutrition examination Surveys (NHANES I-NCHS, 1973) conducted by the National Center for Health Statistics from 1971 to 1975. NHANES I is a stratified multi-stage clustered probability survey of the non-institutionalized population of the United States.8 Trained personnel recorded among other variables the height and weight of examinees. A follow-up study (NCHS, 1992) traces the survivors of NHANES I into the early 1990s, and therefore we are able to determine who died within two decades of the examination. In addition, the cause of death for non-survivors was recorded.
The sub-sample of UA veterans included in the present study consists of 9509 war survivors that were examined after 1865 between the ages of 45 and 64 for whom there is information on their date of death.9 For comparative purposes, the NHANES I sub-sample was restricted to men in the same age range and includes 1912 examinees.10 Both sub-samples were further subdivided into two groups. At some stages of the analysis, the Union Army sample was restricted to veterans who were examined after 1890. In this year, the rules for entry into the pension system were liberalized through the Dependent Pension Act, and any veteran who had honorably served for ninety days and was disabled for manual labor was eligible for a pension (US Bureau of Pensions, 1925).11 The number of pensioners soared after 1890. In order to keep genetic and ethnic characteristics constant, portions of the analysis restrict NHANES I to non-Hispanic white men.12 Finally, the cause of death was recorded for sub-samples of the UA (n = 6321) and NHANES I (n = 1837) who died within 20 years after the medical examination.13
4. BMI and mortality: the changing role
Over the past century, the risk of having a higher BMI has decreased for middle-aged American men. The comparison between UA veterans and NHANES I cohorts reveals that there has been an upward shift in healthy BMI. Nowadays individuals with higher BMIs are healthier than their counterparts a century earlier. Underweight and extremely obese contemporary men, however, are at a higher relative risk than their ancestors.
Fig. 1a–d show the incidence rate of death of UAveterans and NHANES I examinees censored 20 years after the medical examination. There are five possible BMI classes with 5 unit intervals—BMI < 20, 20 ≤ BMI < 25, 25 ≤ BMI < 30, 30 ≤ BMI < 35, and BMI ≥ 35. The samples were further subdivided into 5-year age groups.
Fig. 1.
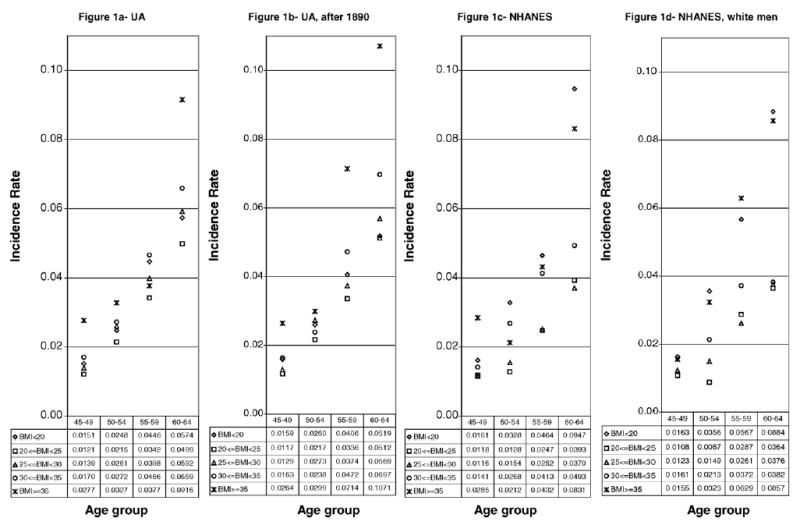
Incidence rate of death for UA and NHANES I men by BMI and age groups (censored 20 years after medical examination). Note: The incidence rate was calculated as the number of deaths/person-years.
For UA veterans, a BMI between 20 and 25 was clearly the healthiest for all age groups. Fig. 1a presents all men who survived the war and that were examined between the ages of 45 and 64. Fig. 1b, on the other hand, restricts the sample to veterans that were examined after the general law of 1890. Both figures follow a similar pattern. The inclusion of all those examined before 1890 does not make a big difference in the general trend. Veterans with a BMI between 20 and 25 had the lowest incidence rate. Conversely, the most likely to die were those with a BMI of 35 and above (except for the 55–59 age group in Fig. 1a). Veterans with a BMI between 30 and 35 were the second group at highest risk of death. In general terms, it was healthier to have a BMI below 20 than a BMI of 30 or larger. Men with a BMI in the 25–30 range were at a higher risk than the 20–25 group, but were in general healthier than most other groups. Arguably, men with a BMI of 25 were at a higher risk of death than those under 25.
In NHANES I, a BMI between 20 and 25 was not consistently the healthiest for all age groups. In some cases, examinees with a BMI in the 25–30 group were at a similar or even lower risk of death. In Fig. 1c and d, we can observe the incidence rate of death for men in NHANES I. Fig. 1c corresponds to all men between the ages of 45 and 64 years, while Fig. 1d displays only non-Hispanic white men within the same age range. Both groups of men present similar trends. The healthiest men appear to be those with a BMI of 20–25 and those with a BMI of 25–30 at the time of the interview. Clearly, men with a BMI of 30–35 were unhealthier than examinees with a BMI between 20 and 30. The worst possible situation was to have a BMI below 20 or a BMI of 35 or larger. In other words, underweight and very obese people had significantly shorter lifespans.
Not surprisingly, men from NHANES I had higher survival rates than their counterpart a century earlier. For instance, 20 years after the first examination, 62% of the healthiest examinees in NHANES I—that is men with a BMI between 20 and 25 were alive. Comparatively, only 54% of the Union Army veterans with similar BMI characteristics had survived. This group (20 ≤ BMI < 25) remained the healthiest for both time periods. Nevertheless, the importance of other BMI categories shifted through time as a determinant of longevity. First, the difference between those with a BMI between 25 and 30 and those with a BMI between 20 and 25 deserves notice in the UA sample. For the NHANES I cohort the difference practically vanished, such that it is no longer clear that one category is healthier than the other. Second, being underweight (BMI < 20) became riskier relative to the other categories over time. For the UA it was riskier to be extremely obese (BMI ≥ 35) than being underweight, whilst for men in NHANES I both situations were equally risky.
In Fig. 2 we can further observe the differences in survival rates by BMI class and the changes in risk through time. The figure presents the mean relative risk of death within 20 years following the first medical examination.14 Once again we can see that Union Army veterans with a BMI between 25 and 30 were at a higher risk than those in the normal range (20 ≤ BMI < 25). The difference in risk between both BMI categories withered away for NHANES I examinees. Fig. 2 also shows a U-shaped relationship between risk and BMI – such that risk increases at low and high BMI levels – a pattern that has been found by previous research (Fogel, 1993, 1994; Kim, 1996; Waaler, 1984). Note, however, that the relative risk by BMI class is steeper for NHANES I than for the UA. Underweight men in NHANES I were on average almost 50% more likely to die in relative terms than underweight UA veterans. Even in absolute terms, underweight men in NHANES I appeared to have a higher risk than the UA sample (see Table A.4).15 Extreme obesity was also riskier for NHANES I examinees, especially for non-Hispanic white men. This result may be questionable, given that only a very small number of examinees in both samples were extremely obese, and therefore the confidence intervals are very large and not statistically distinguishable.
Fig. 2.
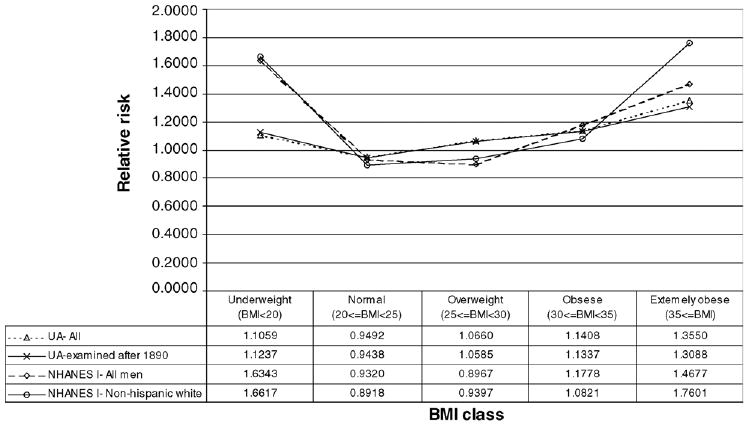
Relative risk of death within 20 years of examination for men ages 45–64, 1870–1992.
Table A4.
Absolute mean risk of death within 20 years of examination by BMI class for men ages 45–64
| UA
|
NHANES I
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All
|
Examined after 1890
|
All
|
Non-Hispanic white
|
|||||||||
| 95% CI
|
95% CI
|
95% CI
|
95% CI
|
|||||||||
| BMI class | Mean | LB | UB | Mean | LB | UB | Mean | LB | UB | Mean | LB | UB |
| Underweight (BMI < 20) | 0.5401 | 0.5109 | 0.5693 | 0.5614 | 0.5176 | 0.6051 | 0.5920 | 0.4754 | 0.7087 | 0.5874 | 0.4513 | 0.7235 |
| Normal (20 ≥ BMI < 25) | 0.4636 | 0.4511 | 0.4760 | 0.4715 | 0.4537 | 0.4893 | 0.3376 | 0.2908 | 0.3844 | 0.3153 | 0.2568 | 0.3737 |
| Overweight (25 ≥ BMI < 30) | 0.5206 | 0.4976 | 0.5436 | 0.5288 | 0.4987 | 0.5589 | 0.3248 | 0.2755 | 0.3742 | 0.3322 | 0.2836 | 0.3808 |
| Obese (30 ≥ BMI < 35) | 0.5571 | 0.5048 | 0.6094 | 0.5664 | 0.5013 | 0.6315 | 0.4266 | 0.3346 | 0.5187 | 0.3825 | 0.2890 | 0.4761 |
| Extremely obese (35 ≥ BMI) | 0.6618 | 0.5464 | 0.7771 | 0.6538 | 0.5201 | 0.7876 | 0.5317 | 0.3561 | 0.7072 | 0.6222 | 0.3921 | 0.8523 |
| Sample mean | 0.4884 | 0.4783 | 0.4984 | 0.4996 | 0.4855 | 0.5137 | 0.3622 | 0.3288 | 0.3957 | 0.3535 | 0.3179 | 0.3891 |
A breakdown into two unit BMI intervals further shows the U-shaped relationship between BMI and risk of death. Fig. 3 displays the mean relative risk of death within 20 years of the first examination by two unit BMI groups. Once again the association between BMI and risk appears to be steeper for NHANES I than for the UA. In addition, for Union Army veterans a BMI between 22 and 24 had the lowest risk, closely followed by the 20–22 and 24–26 ranges. The minimum risk for the UA sample is situated at a plateau between 20 and 26. Conversely, the minimum risk for NHANES I appears in the 26–28 BMI interval, closely followed by the 22– 24 and 24–26 intervals, therefore forming a plateau between 22 and 28 BMI points.16 In fact, fitting a cubic function shows that the minimum risk shifted by almost 3 BMI points between both time periods, from 23.13 for the UA to 25.99 for NHANES I. Nevertheless, the cubic fit may be too restrictive by forcing the data into a curvilinear function, and actually erases the risk plateaus for both time periods.17 Figs. 2 and 3 show that Waaler curves may be getting steeper over time, given that extreme values are becoming relatively riskier.
Fig. 3.
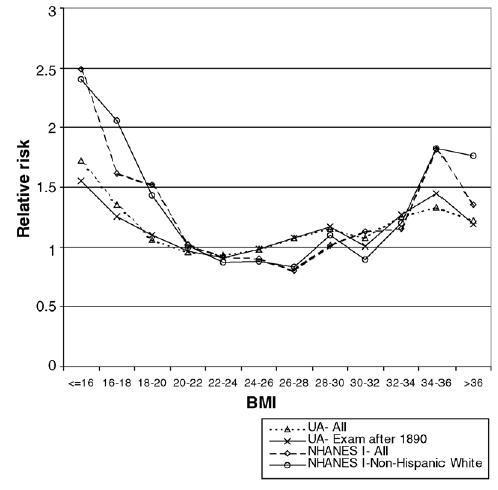
Relative risk of death within 20 years of exam by BMI for men ages 45–64.
The upward shift in healthy BMI from the UA to NHANES can be further evidenced in Table 3, where a Cox proportional hazard model is presented. The regression compares the hazard ratios for different BMI classes (by 5 unit intervals) against a BMI range of 20–25 (the omitted category). For the cohort born during the first half of the 19th century, having a BMI between 20 and 25 was the healthiest situation. Although veterans with a BMI of 25–30 were the second healthiest group, they were clearly at a higher risk of death than those in the normal range. This group was closely followed in risk by those with a BMI lower than 20. The veterans with the highest death risk were those with a BMI of 30 or more, especially those extremely obese who had an average hazard ratio 66% higher than those with a BMI between 20 and 25. Comparatively, the men in NHANES I with a BMI of 25–30 were as healthy as those with a BMI between 20 and 25.18 The relative risk of the other BMI classes in relation to those with a BMI between 20 and 30 is much higher for NHANES I examinees than for the UA, a finding that is consistent with the curves shown in Figs. 2 and 3 above. A further breakdown (see Table A.5 in Appendix A) into two unit intervals shows that the lowest risk for the UA was in the 20–26 BMI range, while the lowest risk for NHANES appears for men with a BMI between 22 and 28 at the time of the interview.19 The regression includes a linear term for age to control for the effect of aging, as expected, each additional year increased the risk of death.20
Table 3.
Cox Proportional Hazard Model- Output variable: years lived at first examination (censored at 20 years)
| UA- All
|
UA- Exam after 1890
|
NHANES I- All
|
NHANES I-Non-Hispanic white
|
|||||
|---|---|---|---|---|---|---|---|---|
| Covariates | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| |
| Age | 1.0892 | 0.0000 | 1.0893 | 0.0000 | 1.0936 | 0.0000 | 1.0938 | 0.0000 |
| BMI dummies = 1 if | ||||||||
| BMI < 20 | 1.2021 | 0.0000 | 1.1688 | 0.0210 | 2.1304 | 0.0000 | 2.3955 | 0.0000 |
| 25 ≤ BMI < 30 | 1.1454 | 0.0000 | 1.1332 | 0.0100 | 1.0385 | 0.7210 | 1.1287 | 0.3490 |
| 30 ≤ BMI < 35 | 1.2635 | 0.0020 | 1.2656 | 0.0100 | 1.6035 | 0.0020 | 1.4890 | 0.0370 |
| 35 ≤ BMI | 1.6629 | 0.0010 | 1.8372 | 0.0000 | 1.9807 | 0.0030 | 2.5657 | 0.0000 |
| n | 9444 | 4817 | 1871 | 1219 | ||||
| Prob > χ2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||
Note: P > |z| calculated using robust standard errors. Omitted category 20 ≤ BMI < 25.
Table A5.
Cox proportional hazard model—output variable: years lived after first examination (censored at 20 years)
| UA—all
|
UA—exam after 1890
|
NHANES I—all
|
NHANES I—non-Hispanic white
|
|||||
|---|---|---|---|---|---|---|---|---|
| Covariates | Haz. ratio | P > |z| | Haz. ratio | P > |z| | Haz. ratio | P > |z| | Haz. ratio | P > |z| |
| Age | 1.0890 | 0.0000 | 1.0893 | 0.0000 | 1.0938 | 0.0000 | 1.0957 | 0.0000 |
| BMI dummies = 1 if: | ||||||||
| BMI < 16 | 2.9114 | 0.0000 | 1.9354 | 0.4470 | 6.8610 | 0.0000 | 7.8163 | 0.0000 |
| 16 ≥ BMI < 18 | 1.4999 | 0.0000 | 1.2942 | 0.1590 | 2.4380 | 0.0090 | 3.7987 | 0.0000 |
| 18 ≥ BMI < 20 | 1.1236 | 0.0460 | 1.1404 | 0.1090 | 1.7489 | 0.0020 | 1.9055 | 0.0060 |
| 20 ≥ BMI < 22 | 1.0004 | 0.9930 | 1.0239 | 0.7090 | 1.1353 | 0.4970 | 1.3552 | 0.1740 |
| 22 ≥ BMI < 24 | 0.9645 | 0.4380 | 0.9583 | 0.4920 | 0.9595 | 0.7890 | 1.0045 | 0.9820 |
| 26 ≥ BMI < 28 | 1.1606 | 0.0160 | 1.1901 | 0.0250 | 0.8818 | 0.4000 | 1.0222 | 0.9040 |
| 28 ≥ BMI < 30 | 1.2784 | 0.0020 | 1.3262 | 0.0030 | 1.3456 | 0.0690 | 1.6023 | 0.0160 |
| 30 ≥ BMI < 32 | 1.1666 | 0.1390 | 1.1073 | 0.4310 | 1.5368 | 0.0520 | 1.2412 | 0.4000 |
| 32 ≥ BMI < 34 | 1.3918 | 0.0110 | 1.4020 | 0.0390 | 1.5657 | 0.0590 | 1.8426 | 0.0420 |
| 34 ≥ BMI < 36 | 1.4546 | 0.0210 | 1.8195 | 0.0000 | 2.2698 | 0.0040 | 2.6554 | 0.0130 |
| 36 ≥ BMI | 1.5300 | 0.0370 | 1.7722 | 0.0130 | 1.9229 | 0.0170 | 2.9268 | 0.0000 |
| n | 9444 | 4817 | 1871 | 1219 | ||||
| Prob > χ2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||
Note: P > |z| calculated using robust standard errors. Omitted category 24 ≥ BMI < 26.
Given that age is such an important determinant of survivorship, the samples were broken down into 10-year age intervals. Tables 4a and 4b show the results for the proportional hazard models of theses subdivisions. A BMI under 20 was riskier than the 20–25 BMI group for all age groups, but even riskier in the case of NHANES I. Once again, veterans with a BMI between 25 and 30 were at higher risk than the benchmark group (20 ≤ BMI < 25), a result that does not hold for NHANES I. Finally, having a BMI of 30 or larger increased the risk of death for both the UA and NHANES I in comparison to those with a BMI between 20 and 25. As we can see breaking down the samples into age groups, still reveals the general pattern that the risk of having a BMI between 25 and 30 has become less risky over time.
Table 4a.
Cox proportional hazard model on ua veterans—output variable: years lived after first examination (censored at 20 years)
| All
|
Examined after 1890
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Ages 45–54
|
Ages 55–64
|
Ages 45–54
|
Ages 55–64
|
|||||
| Covariate | Haz. ratio | P > |z| | Haz. ratio | P > |z| | Haz. ratio | P > |z| | Haz. ratio | P > |z| |
| Age | 1.1017 | 0.0000 | 1.0783 | 0.0000 | 1.1086 | 0.0000 | 1.0791 | 0.0000 |
| BMI dummies = 1 if: | ||||||||
| BMI < 20 | 1.1887 | 0.0060 | 1.2168 | 0.0020 | 1.2536 | 0.0200 | 1.1004 | 0.3020 |
| 25 ≤ BMI < 30 | 1.1507 | 0.0050 | 1.1416 | 0.0130 | 1.1641 | 0.0250 | 1.1027 | 0.1560 |
| 30 ≤ BMI < 35 | 1.2420 | 0.0260 | 1.2952 | 0.0230 | 1.1778 | 0.2180 | 1.3582 | 0.0180 |
| 35 ≤ BMI | 1.7714 | 0.0020 | 1.5355 | 0.0370 | 1.6681 | 0.0120 | 2.3045 | 0.0020 |
| n | 6404 | 3040 | 3128 | 1689 | ||||
| Prob > χ2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||
Note: P > |z| calculated using robust standard errors. Omitted category 20 ≤ BMI < 25.
Table 4b.
Cox proportional hazard model on NHANES I examinees—output variable: years lived after first examination (censored at 20 years)
| All
|
Non-Hispanic white
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Ages 45 to 54
|
Ages 55 to 64
|
Ages 45 to 54
|
Ages 55 to 64
|
|||||
| Haz. ratio | P > |z| | Haz. ratio | P > |z| | Haz. ratio | P > |z| | Haz. ratio | P > |z| | |
| Age | 1.0465 | 0.1000 | 1.0915 | 0.0000 | 1.0309 | 0.3890 | 1.0674 | 0.0070 |
| BMI dummies = 1 if | ||||||||
| BMI < 20 | 1.9569 | 0.0090 | 2.2985 | 0.0000 | 2.2585 | 0.0220 | 2.3479 | 0.0000 |
| 25 ≤ BMI < 30 | 1.0965 | 0.6050 | 0.9741 | 0.8450 | 1.3999 | 0.1400 | 0.9729 | 0.8650 |
| 30 ≤ BMI < 35 | 1.6866 | 0.0280 | 1.5498 | 0.0340 | 1.9366 | 0.0300 | 1.2380 | 0.3850 |
| 35 ≤ BMI | 1.9080 | 0.0970 | 2.1125 | 0.0090 | 2.7338 | 0.0200 | 2.6932 | 0.0010 |
| n | 1035 | 836 | 674 | 545 | ||||
| Prob > χ2 | 0.0174 | 0.0000 | 0.0633 | 0.0000 | ||||
Note: P > |z| calculated using robust standard errors. Omitted category 20 ≤ BMI < 25.
We should take into consideration that the analysis was done looking at all-cause mortality and not the emergence of particular morbidities. The risk of specific diseases associated with overweight and obesity may be different than those related to death as the final outcome. Nevertheless, it is possible to further analyze the interaction between different BMI levels and the specific cause of death.21 Tables 5a and 5b show the results for the Cox proportional hazard competing risk models for the UA and NHANES I. The sample was restricted to men with a known cause of death, excluding those who died of external causes such as accidents. The causes of death were classified into four categories: infectious diseases, cancer, cardiovascular diseases (CVD) and other.22 The tables also present the results for all-cause mortality.
Table 5a.
Cox proportional hazard competing risk model on UA veterans—output variable: years lived after first examination (censored at 20 years)
| All cause
|
Infectious
|
Cancer
|
CVD
|
Other
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All
|
Exam after 1890
|
All
|
Exam after 1890
|
All
|
Exam after 1890
|
All
|
Exam after 1890
|
All
|
Exam after 1890
|
|||||||||||
| Covariates | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| |
| Age | 1.0961 | 0.0000 | 1.1059 | 0.0000 | 1.0614 | 0.0000 | 1.0682 | 0.0000 | 1.0811 | 0.0000 | 1.1067 | 0.0000 | 1.1143 | 0.0000 | 1.1414 | 0.0000 | 1.1067 | 0.0000 | 1.1006 | 0.0000 |
| BMI dummies = 1 if: | ||||||||||||||||||||
| BMI < 20 | 1.2272 | 0.0130 | 1.1013 | 0.4490 | 2.0320 | 0.0000 | 2.2728 | 0.0000 | 1.1908 | 0.5470 | 0.9722 | 0.9480 | 0.6631 | 0.0390 | 0.5805 | 0.0860 | 1.1747 | 0.2240 | 0.9766 | 0.9070 |
| 25 ≤ BMI < 30 | 1.2293 | 0.0020 | 1.2093 | 0.0350 | 0.8392 | 0.2670 | 1.1488 | 0.5090 | 1.5137 | 0.0570 | 1.5368 | 0.1180 | 1.6521 | 0.0000 | 1.6346 | 0.0020 | 1.1074 | 0.3510 | 0.9057 | 0.5160 |
| 30 ≤ BMI < 35 | 1.5782 | 0.0000 | 1.5946 | 0.0020 | 0.7923 | 0.4900 | 0.8554 | 0.7330 | 2.6040 | 0.0040 | 2.8794 | 0.0060 | 1.7396 | 0.0090 | 1.5620 | 0.1220 | 1.7332 | 0.0030 | 1.6871 | 0.0220 |
| 35 ≤ BMI | 1.9763 | 0.0110 | 2.0491 | 0.0230 | – | – | – | – | 4.3893 | 0.0000 | 5.5372 | 0.0000 | 1.9582 | 0.1180 | 1.5557 | 0.4430 | ||||
| n | 6321 | 3153 | 6321 | 3153 | 6321 | 3153 | 6321 | 3153 | 6321 | 3153 | ||||||||||
| Prob > χ2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||||
Note: P > |z| calculated using robust standard errors. Omitted category 20 ≤ BMI < 25. Cells with no observations are portrayed by a dash (–).
Table 5b.
Cox proportional hazard competing risk model on NHANES I examinees—output variable: years lived after first examination (censored at 20 years)
| All cause
|
Infectious
|
Cancer
|
CVD
|
Other
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All
|
Non-Hispanic white
|
All
|
Non-Hispanic white
|
All
|
Non-Hispanic white
|
All
|
Non-Hispanic white
|
All
|
Non-Hispanic white
|
|||||||||||
| Covariates | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| | Haz. Ratio | P > |z| |
| Age | 1.0936 | 0.0000 | 1.0933 | 0.0000 | 1.1127 | 0.0810 | 1.0509 | 0.4030 | 1.0800 | 0.0000 | 1.0855 | 0.0000 | 1.0809 | 0.0000 | 1.0875 | 0.0000 | 1.1442 | 0.0000 | 1.1261 | 0.0000 |
| BMI dummies = 1 if: | ||||||||||||||||||||
| BMI < 20 | 2.1740 | 0.0000 | 2.4410 | 0.0000 | 1.6478 | 0.5720 | 2.3918 | 0.3520 | 1.7288 | 0.0960 | 1.5552 | 0.3360 | 1.1770 | 0.6410 | 1.7232 | 0.1810 | 4.6021 | 0.0000 | 4.7269 | 0.0000 |
| 25 ≤ BMI < 30 | 1.0353 | 0.7500 | 1.1268 | 0.3710 | 0.8003 | 0.7400 | 0.8529 | 0.8300 | 0.9786 | 0.9120 | 1.0343 | 0.8850 | 1.3935 | 0.0430 | 1.6883 | 0.0110 | 0.5725 | 0.0210 | 0.5444 | 0.0430 |
| 30 ≤ BMI < 35 | 1.6293 | 0.0020 | 1.5254 | 0.0300 | 1.1492 | 0.9020 | 6.1E–17 | 0.0000 | 1.6716 | 0.1160 | 1.2426 | 0.5390 | 2.0670 | 0.0010 | 2.6608 | 0.0000 | 0.9312 | 0.8630 | 0.5704 | 0.4180 |
| 35 ≤ BMI | 2.0562 | 0.0020 | 2.6779 | 0.0000 | 1.6E–15 | 0.0000 | 5.8E–17 | 0.0000 | 1.8234 | 0.1970 | 3.0379 | 0.0170 | 3.3098 | 0.0000 | 4.0046 | 0.0000 | 0.5452 | 0.3370 | 0.7450 | 0.6830 |
| n | 1837 | 1197 | 1837 | 1197 | 1837 | 1197 | 1837 | 1197 | 1837 | 1197 | ||||||||||
| Prob > χ2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||||
Note: P > |z| calculated using robust standard errors. Omitted category 20 ≤ BMI < 25.
All-cause mortality remains relatively similar to the results presented in Table 4 when restricting the sample to examinees with a known cause of death. The basic conclusion that men in the UA with a BMI of 25–30 have a higher risk than those with a BMI in the 20–25 range still holds, and the difference between these two categories in NHANES I is still non-existent with respect to mortality. In addition, men in NHANES I with extreme BMIs (below 20 and above 35) were at a higher relative risk of death than their UA counterpart. Note, however, that UA examinees with a BMI below 20 are not at a higher risk than the 20–25 group. Although the hazard ratio is larger than 1, indicating a higher probability of death, the low statistical significance makes the result questionable.
Death from infectious diseases declined substantially since the Civil War. As the epidemiologic transition progressed, the relative number of deaths from infectious diseases declined giving way to a higher percent of deaths from chronic degenerative diseases.23 In the case of UA veterans with a known cause of death, 23% died from infectious diseases within the first 20 years after the medical examination. Comparatively, only less than 3% of NHANES I examinees died from such cause. Being underweight increased the odds of dieing from infectious diseases in the past. Underweight veterans (BMI < 20) were twice as likely to die from infectious diseases than those in the 20–25 BMI range.24 Underweight NHANES I examinees, on the other hand, were as likely to die than their 20–25 BMI counterpart.25 In addition, men in both samples with a BMI of 25 or more had the same risk of death from infectious diseases as the 20–25 BMI group.
In the recent years, the association between adiposity and a higher risk of cancer has been well documented (Calle et al., 2003). UA veterans show a clear relationship between higher BMI and death from cancer. Veterans with a BMI of 25 or higher had a higher probability of death from cancer than those with a lower BMI, and the risk keeps increasing as BMI raises, such that veterans with a BMI between 30 and 35 were almost three times as likely to die from cancer than those in the 20–25 BMI range. On the other hand, having a BMI under 20 had no effect on the odds of cancer in the UA. For NHANES I having a BMI between 25 and 30 did not increase the probability of dieing from cancer in comparison to the 20–25 BMI group. In addition, there was a weak relationship between having a BMI over 30 and death from cancer. For all men, having a BMI over 30 increased the risk of cancer as the cause of death. For non-Hispanic whites, a BMI over 30 increased the odds of death from cancer, however, the statistical significance is not as satisfactory to solidly claim a higher risk.26 Yet, a BMI of 35 or more increased over three times the risk of death from cancer for non-Hispanic whites. Note also that for all men in NHANES I, the risk of death from cancer increased with a BMI under 20, an effect that was not clear for non-Hispanic men. Underweight men may have been more prone to death from cancer because of the disease itself. Given that many cancer patients’ suffer from cachexia and, therefore, loose weight and body mass.
Several studies demonstrate that a higher fat concentration increases the risk of cardiovascular diseases (CVD) and death from CVD (Gordon and Kannel, 1976; Hubert et al., 1983; Tuomilehto et al., 1987; Lee et al., 1993; Calle et al., 1999; Jonsson et al., 2002). The link between obesity and CVD has been one of the main concerns among epidemiologists and public health specialists to recommend healthier BMI levels across populations. Accordingly, in the UA and NHANES I samples, the risk of death from CVD increases with higher levels of BMI. The risk of CVD death in both samples augments with a BMI of 25 or more. Furthermore, the risk intensifies as we move from a BMI category to a higher one. The effect of being underweight is not clear when we look at both samples. While in the UA, having a BMI under 20 reduced the risk of death from CVD, for NHANES I examinees there seems to be no consequence.27
Over two fifths of the deaths catalogued as “other” in the UA were from respiratory diseases and over a quarter died from genitourinary problems. Digestive disorders caused close to 17% of these deaths. Comparatively, almost half of the deaths labeled as “other” in NHANES I were from diseases of the respiratory system and around a fifth from diseases of the digestive system. The other two leading causes in order of importance were endocrine, nutritional and metabolic diseases, and immunity disorders and diseases of the nervous system and sense organs. In the UA, men with a BMI of 25–30 were at the lowest risk of death from respiratory disease, while being underweight had no impact. In NHANES I men with a BMI 25–30 were also at the lowest risk of death from respiratory diseases. Men with a BMI under 20 were six times as likely to die from respiratory disease than those in the 20–25 BMI group, to a large degree explaining the huge hazard ratio for underweight examinees who died from “other” causes (Table 5b). BMI differences had no consequence on the odds of death from digestive disorders in either the UA or NHANES I.28 Death from endocrine, nutritional and metabolic diseases, and immunity disorders increased substantially for veterans with a BMI of 20 or higher, and the risk increased with higher BMI levels, such that men with a BMI of 35 or higher were eight times as likely to die from these diseases than those in the 20–25 BMI range. Although a very small number of examinees (n = 13) in NHANES I died from endocrine, nutritional and metabolic diseases, and immunity disorders, underweight (BMI < 20) men and men with a BMI over 30 were at a much higher risk than the 20–25 BMI group.29 Yet, the 25–30 BMI group was not at a higher risk. Finally, men with a BMI 35 or higher in the UA had a higher risk of death deaths from genitourinary diseases. Underweight veterans were at a lower risk of death form this cause, particularly in the case of those examined after 1890. Very few examinees died in NHANES I from genitourinary diseases to make a reliable comparison.30
5. Discussion
American white men living during the last third of the 20th century had different BMI cut-off risk limits than their counterpart at the end of the 20th century. Over the course of the past decades, the BMI cut-off points that define overweight and obesity may have grown, which in turn may imply a long-term secular shift toward healthier bodies at higher BMI levels. A plausible explanation for the expansion in BMI risk limits is the amount of body fat stored for the same BMI level. As stated earlier, BMI is used as a predictor of body fat concentration: higher amounts of adipose tissue increase the risks of morbidity and mortality. The association between BMI and percent body fat has been well documented in a number of studies (Womersley and Durnin, 1977; Norgan and Ferro-Luzzy, 1985; Garrow and Webster, 1985; Deurenberg et al., 1991; Gallager et al., 1996). Yet, different BMI levels may predict different body fat by gender and age. A second important finding is that extreme BMI’s may be riskier in contemporary populations than they were in the past. In other words, Waaler curves may have steep ended in the modern era.
The upward shift in BMI risk limits over time for American men may be the result of changes in the human frame. While there is no way to test differences in body complexion beyond BMI during the late 19th century with the present sample, according to Costa (2004), since the 19th century the body frame of American men has increased in terms of height and weight.31 Yet, the amount of abdominal fat has decreased. In the past, men had higher concentrations of body fat for the same BMI than their contemporaries. In the case of NHANES I the evidence suggests that the amount of fat mass in the body decreased monotonically the probability of survival for the same BMI level. Conversely, the amount of fat free mass (mainly muscle) increased the probability of survival (Allison et al., 2002). Additionally, other anthropometric changes may have occurred such as a change in the relative leg length to frame size.32 Thus, making contemporaries leaner for the same BMI.
The change in survivorship for similar BMI levels through time may respond to physiological transformations that are not determined by differences in ethnicity or genetics. Fogel and Costa coined the term technophysio evolution to describe the “synergism between technological and physiological improvements [that] has produced a form of human evolution that is biological but not genetic, rapid, culturally transmitted, and not necessarily stable” (Fogel, 2000b: 74). Technophysio evolution, unlike genetic theory, only applies to the past three 100 years of human history, and in particular to the past century when the human body increased in size and has become more robust with a stronger vital organ system.
One of the trademarks of such transformation is the increase in adult stature. Populations throughout the world have grown taller over the past three centuries as a reflection of better nutrition and less disease. Differences in body build and height can be appreciated across time for similar ethnicities. The populations of many countries have grown taller and gained body mass.33 Changes in heights have been observed for populations that have had little genetic and ethnic change, such as in Sweden (Sandberg and Steckel, 1997).
Taller people tend to live longer because their adult stature is a product of their nutritional and disease history during their developmental years.34 In a study of Norwegian mortality, Kim (1996) found that for the same BMI levels shorter people lived less.35 A similar result can be found for the UA and NHANES I as shown in Tables 6a and 6b. Both tables present the absolute and relative risk of death within a 20-year interval by height and BMI for each sample respectively. The sample sizes are very small and therefore some of the cells have few observations or none. Nevertheless, it is possible to appreciate the general trend: taller men had a higher probability of survival for the same BMI range. For example, in Table 6a we can see that for UA veterans who had a BMI between 20 and 25 their risk of death decreased progressively for higher statures. Men who were between 1.5 and 1.6 m had a relative risk of death of 1.191, while men between 1.9 and 2 m had a relative risk of death of 0.819. Correspondingly, Table 6b shows a similar pattern for men within different groups of BMI.
Table 6a.
Risk of death within 20 years of examination (UA—all) men ages 45–64
| BMI (kg/m2) class
|
||||||
|---|---|---|---|---|---|---|
| Height (m) | BMI < 20 | 20 ≤ BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | 35 ≤ BMI | Total |
| <1.50 | 1.000 | 0.000 | 0.000 | 1.000 | 0.571
1.170 |
|
| 0 | 3 | 1 | 2 | 1 | 7 | |
| 1.5 | 0.545 | 0.582 | 0.516 | 0.495 | 0.458 | 0.525 |
| 1.117 | 1.191 | 1.057 | 1.014 | 0.938 | 1.076 | |
| 11 | 110 | 184 | 103 | 24 | 432 | |
| 1.6 | 0.609 | 0.506 | 0.557 | 0.719 | 0.750 | 0.532 |
| 1.247 | 1.036 | 1.139 | 1.472 | 1.536 | 1.090 | |
| 248 | 1692 | 469 | 64 | 12 | 2485 | |
| 1.7 | 0.524 | 0.445 | 0.511 | 0.524 | 0.786 | 0.471 |
| 1.072 | 0.911 | 1.046 | 1.073 | 1.609 | 0.963 | |
| 634 | 3451 | 953 | 145 | 28 | 5211 | |
| 1.8 | 0.533 | 0.440 | 0.481 | 0.636 | 0.667 | 0.467 |
| 1.091 | 0.901 | 0.984 | 1.303 | 1.365 | 0.956 | |
| 214 | 879 | 206 | 33 | 3 | 1335 | |
| 1.9 | 0.231 | 0.400 | 0.833 | 0.333 | 0.405 | |
| 0.473 | 0.819 | 1.706 | 0.683 | 0.830 | ||
| 13 | 15 | 6 | 3 | 0 | 37 | |
| ≥2 | 0.000 | 0.000 | ||||
| 0.000 | 0.000 | |||||
| 2 | 0 | 0 | 0 | 0 | 2 | |
| Total | 0.540 | 0.464 | 0.521 | 0.557 | 0.662 | 0.488 |
| 1.106 | 0.949 | 1.066 | 1.141 | 1.355 | 1.000 | |
| 1122 | 6150 | 1819 | 350 | 68 | 9509 | |
Note: Each cell gives the 20-year relative risk of mortality, 20-year (absolute) mortality risk, and the number of observations.
Table 6b.
Risk of death within twenty years of examination (NHANES I)- men ages 45–64
| BMI (kg/m2) class
|
||||||
|---|---|---|---|---|---|---|
| Height (m) | BMI < 20 | 20 ≤ BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | 35 ≤ BMI | Total |
| <1.50 | 1.000 | 1.000 | 1.000 | |||
| 2.761 | 2.761 | |||||
| 1 | 1 | 0 | 0 | 0 | 2 | |
| 1.5 | 0.648 | 0.043 | 0.550 | 0.424 | 0.442 | |
| 1.790 | 0.119 | 1.518 | 1.171 | 0.000 | 1.221 | |
| 6 | 8 | 17 | 8 | 1 | 40 | |
| 1.6 | 0.716 | 0.429 | 0.353 | 0.341 | 0.000 | 0.396 |
| 1.975 | 1.185 | 0.974 | 0.943 | 0.000 | 1.094 | |
| 32 | 150 | 227 | 61 | 11 | 481 | |
| 1.7 | 0.545 | 0.328 | 0.326 | 0.518 | 0.336 | 0.370 |
| 1.506 | 0.904 | 0.900 | 1.430 | 0.927 | 1.022 | |
| 64 | 369 | 446 | 120 | 28 | 1027 | |
| 1.8 | 0.497 | 0.286 | 0.281 | 0.265 | 0.567 | 0.298 |
| 1.373 | 0.790 | 0.775 | 0.731 | 1.566 | 0.822 | |
| 22 | 129 | 143 | 42 | 7 | 343 | |
| 1.9 | 0.000 | 0.187 | 0.000 | 0.166 | 0.855 | 0.091 |
| 0.000 | 0.516 | 0.000 | 0.459 | 2.359 | 0.251 | |
| 1 | 9 | 6 | 3 | 0 | 19 | |
| ≥2 | ||||||
| 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0.592 | 0.338 | 0.325 | 0.427 | 0.532 | 0.362 |
| 1.634 | 0.932 | 0.897 | 1.178 | 1.468 | 1.000 | |
| 126 | 666 | 839 | 234 | 47 | 1912 | |
Note: Each cell gives the 20-year relative risk of mortality, 20-year (absolute) mortality risk, and the number of observations.
During the 19th century, the American population was probably the tallest in the world. As Komlos and Baur (2004) point out, Americans did not grow much during the 20th century, especially in comparison to some European populations. Men in NHANES I were on average over 1.5 cm taller than the UA veterans, an increase in stature that explains a small proportion of survivorship differential.36 The increase in mean height between both populations is larger when we look only at men ages 45–49. In this case, UA veterans were close to 2.5 cm shorter than NHANES examinees. Restricting the sample to this age group may give us a more accurate picture of the change in height. Humans get shorter as they age, mainly because the cartilage between joints withers away. People begin to decrease in height during their 50s, and it is difficult to assume that everyone decreases evenly. So by looking at those men examined before age 50 we may be looking at a more accurate description of the adult heights of both cohorts.
While European populations grew substantially taller over the same time period, a 2.5 cm increase is far from negligible. Not only were the UA veterans shorter than NHANES I examinees, but they also had a more spread height distribution. The coefficient of variation, for instance, passed from 0.415 to 0.395. Fig. 7a and b present the density functions for both cohorts. Fig. 4a displays all men ages 45–64, while 4b restricts the sample to ages 45–49. As we can see, the overall NHANES I distribution shifted outward in comparison to the UA, especially for men ages 40–49.37 The shortest 5% of this age group grew by more than 3.6 cm, while the median increased by 2.4 cm. Furthermore, the tallest 95% of men in NHANES were close to 3.8 cm bigger than their UA counterpart.
Fig. 4.
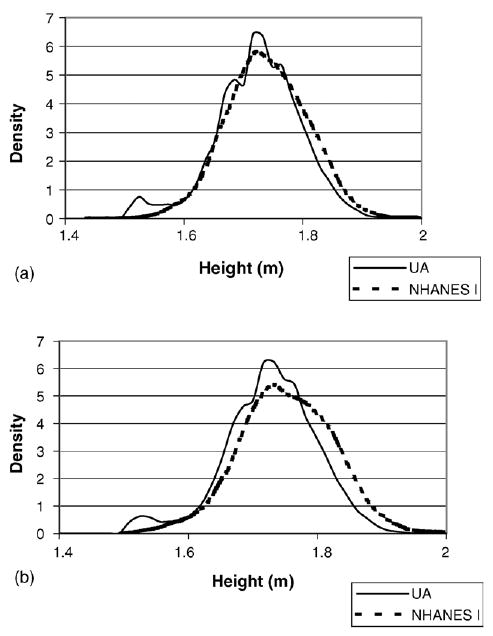
(a) Kernel density funcitons of height in the UA and NHANES I for men ages 45–64. (b) Kernel density funcitons of height in the UA and NHANES I for men ages 45–49.
The mean height of UA veterans decreased as BMI categories increased, while the stature of NHANES I examinees remained more stable across BMI groups. Such difference in mean height by BMI group explains to some degree why the Waaler curves are getting steep ended in modern times. Further research has developed Waaler curves into Waaler surfaces where the simultaneous effect of BMI, height and weight on mortality can be analyzed (Fogel, 1993; Kim, 1996). A key finding from the Waaler surfaces is that the ideal BMI varies with height, such that shorter people tend to be healthier at higher BMIs. As Fogel explains in the case of Norwegians “[a] BMI of 25 is the ideal for men in the neighborhood of 176 cm (69 inches), but for short men (under 168 cm or 66 inches) the ideal BMI is about 26” (Fogel, 2004: 27). Hence stunted individuals—those with a low height for age— need a higher BMI to equalize the risk of death in comparison to taller people. Table 7 presents the mean height and weight by BMI category. The tallest UA army veterans were those with a BMI under 20 and mean height diminished as we move toward higher BMIs, such that those with a BMI of 35 or larger were almost 10 cm shorter than those with a BMI under 20. Comparatively, the differences in mean height across BMI groups for NHANES I examinees are smaller and not monotonically decreasing. So while the mean height for NHANES I examinees remains relatively constant across BMI groups, the height and BMI of UA veterans is inversely related. As a result, underweight veterans were slightly taller than underweight NHANES I examinees and obese veterans were shorter than obese NHANES I examinees. Thus extreme BMIs in the modern cohort are relatively riskier.
Table 7.
Mean height (m) and weight (kg) by BMI class
| UA
|
NHANES I
|
|||||||
|---|---|---|---|---|---|---|---|---|
| All
|
After 1890
|
All
|
Non-Hispanic white
|
|||||
| BMI class | Height | Weight | Height | Weight | Height | Weight | Height | Weight |
| BMI < 20 | 1.742 | 57.587 | 1.738 | 57.381 | 1.723 | 54.713 | 1.721 | 54.690 |
| 20 ≤ BMI < 30 | 1.729 | 66.885 | 1.726 | 66.891 | 1.750 | 70.330 | 1.753 | 70.657 |
| 25 ≤ BMI < 30 | 1.710 | 78.322 | 1.715 | 78.928 | 1.742 | 82.627 | 1.744 | 82.896 |
| 30 ≤ BMI < 35 | 1.673 | 89.628 | 1.679 | 90.317 | 1.734 | 96.046 | 1.736 | 96.119 |
| 35 ≤ BMI | 1.653 | 103.853 | 1.644 | 102.800 | 1.732 | 117.304 | 1.734 | 115.201 |
Another factor that may explain relative riskier extremes in NHANES I is the wider dispersion of BMI in the modern cohort, where a higher percent of the examinees are in the tails of the distribution, especially at the upper tail. We can also see in Table 7 that underweight men in NHANES I are around 3 kg lighter than UA veterans, while overweight NHANES I examinees are around 15 kg heavier than UA veterans with a BMI of 35 or more.
In addition to the overall increase in height it is important to consider that throughout the 19th century adult statures oscillated in the United States. In the three decades that preceded the Civil War, heights declined and mortality increased, while at the same time income per capita income grew rapidly. Some authors have named this paradox of rising incomes and decreasing health the “Antebellum Puzzle” (Steckel, 1995; Komlos, 1996; Haines, 1998). Heights declined during this era because of changes in diet and the deterioration of the disease environment due to urbanization, transport improvements and increased geographic mobility. Nonetheless, during the 20th century adult statures did not oscillate significantly. In fact, there is an upward trend in height for men ages 25–75 in NHANES I by birth cohort, such that men born in the late 1920s are about 1.4 cm shorter on average that men born in the late 1940s. The fact that men who grew up during the 19th century had stature oscillations shows that they grew up under more precarious conditions than men who were born in the 20th century.
UA veterans grew up under unhealthier conditions. A situation that not only reflects in height, but that may also produce less robust vital organs. Further evidence on the robustness of the body can be seen in the age of onset of disease, which has expanded over the past century (Fogel and Canavese, 2004). In other words, people who grew up under healthier conditions may have stronger muscle tissue, heavier organs, and a thus bigger frame for the same stature. As discussed earlier, people with a bigger frame tend to have lower adiposity for the same BMI. Additionally, some of the overweight people in the past may have also suffered from undernutrition, as it is the case today in some developing countries where obesity and undernutrition are found in the same households (Popkin, 2002).
An important component of human development extends back to the womb. The situation under which fetuses grow remains an important determinant of health in infancy and late in life. Some chronic conditions during adulthood, such as cardiovascular disease and non-insulin dependent diabetes, are highly determined by insults in utero.38 The birth weight of term babies has been used as a proxy to in utero conditions and the health status of newborns. Middle-aged people who were born underweight tend to have less glucose tolerance, high blood pressure, and higher rates of obesity (Susser and Stein, 1994).39 In addition, underweight newborns may have more adiposity accumulation as adults (Yajnik, 2004).
There is no information on birth weights during the first half of the 19th century in the United States. Since the mid-19th century, scattered data show that mean birth weights have remained fairly constant (Goldin and Margo, 1989; Ward, 1993; Costa, 1998). The percent of underweight children, however, decreased almost by half between the third quarter of the 19th century and the first third of the 20th century. In their study of newborns in Philadelphia’ Almshouse Hospital, Goldin and Margo (1989) found that 10% of the babies born between 1848 and 1873 were underweight. Additionally, there was a sharp decrease in birth weights for those born between 1866 and 1870. A sign that birth weights also oscillated during the 19th century. Dora Costa argues that 5.5% of the children born in New York’s Lying-In between 1910 and 1931 had low weight.40 While the mean birth weight did not increase considerably ever since, the variance has declined. Disparities in weight between newborns have always been small, but they were larger at the beginning of the 20th century (Costa, 1998).
Given the trends into the past, it is likely that children born during the first half of the 19th century had lower birth weights, had a higher prevalence of low birth weight and more dispersion in birth weight than those born a century later.41 UA army veterans, therefore, were born and grew up under less favorable conditions than NHANES I examinees. During the 1840s, a period when a large number of UA veterans were born, available proteins and calories per capita fell even though agricultural output was growing (Haines et al., 2000).42 In addition, from 1830 to the Civil War the health environment deteriorated as heights and life expectancy declined (Steckel, 1992).
There are of course other factors beyond the developmental years that may explain the upward shift in BMI risk limits. The United States changed spectacularly during the past 200 years. UA veterans lived in a very different world than NHANES examinees did. Lifestyle, diet and the health environment were very different in both time periods. Occupations mechanized, hours of work throughout the life cycle declined, and people use their leisure time very differently.43 As the United States got richer and technology advanced, the amount of food per capita increased and its flow became more constant; it got cheaper, cleaner and healthier. Technological changes such as refrigeration reduced the consumption of salted foods, an important source of hypertension in the past. Cleaning water supplies and installing sewage systems made people less vulnerable to infectious disease. Medical technology also transformed dramatically during the 20th century. Among other developments, NHANES I examinees had the availability of drugs and medical procedures to treat many conditions and reduce the adverse effect of higher BMIs.44
6. Conclusion
The body mass index (BMI) is commonly used as a measure of current nutritional level in humans. Very high and very low BMIs increase the likelihood of morbidity and mortality. High BMIs are often used as predictors of overweight and obesity. Experts around the world have debated over the past years whether or not the same set of risk BMI cut-off points can be applied across populations and ethnicities. As the present paper shows, the cut-off points may be shifting across time. The range of healthiest BMI for American men born during the first half of the 19th century was around two BMI points below those born during the first half of the 20th century. The minimum risk for UA veterans was situated in the 20–26 BMI span, while the minimum risk for NHANES I examinees appeared in the 22–28 BMI range. The findings may imply that differences in BMI risk cut-off points are not only cross ethnic, but also occur across time for similar ethnicities. The dynamics over time in cut-off points suggests that experts should revise the risk limits on a periodic basis in order to transmit the best available information to policy makers and the population.
A plausible explanation for the upward shift in BMI risk cut-off points may be a difference in physiology between both cohorts. In comparison to the NHANES men, a higher proportion of UA veterans may have been born underweight, grew up in unhealthier environments and had less nutrients during their developmental years. As a result, UA veterans were shorter in stature and had less robust vital organs, which may have led to a smaller body frame. People with smaller body frames tend to accumulate more fat for the same BMI than people with larger body frames.
The results by no means imply that we should stop worrying about the obesity epidemic experienced in the United States and the rest world over the past decades, given that not only the mean and median BMIs have increased but also the proportion of people with extreme obesity has risen dramatically. In addition, obesity and extreme obesity have grown at alarming rates since NHANES I was conducted.
Table A2.
Descriptive statistics of age and BMI at measurement for UA veterans by sample
| All (n = 9509)
|
With recorded cause of death within 20 years after exam (n = 6345)
|
|||
|---|---|---|---|---|
| Variable | Mean | S.D. | Mean | S.D. |
| Age at measurement | 52.2 | 5.3 | 51.2 | 4.9 |
| BMI | 23.3 | 3.3 | 23.2 | 3.2 |
Table A3.
Birthplace distribution of UA veterans examined between the ages of 45 to 64 by sample
| Birthplace | All (n = 9509) (%) | With recorded cause of death within 20 years after exam (n = 6345) (%) |
|---|---|---|
| United States | 78.2 | 80.7 |
| Canada | 2.7 | 2.8 |
| British | 3.0 | 2.7 |
| Irish | 4.9 | 4.0 |
| European other | 9.2 | 8.4 |
| Foreign other | 0.1 | 0.1 |
| Missing | 1.9 | 1.3 |
Acknowledgments
I would like to thank Irina Alberro, David Bishai, Joey Burton, Robert Fogel, John Komlos, Lorens Helmchen, participants at the Center for Population Economics workshop, and four anonymous referees. I also acknowledge and thank the support from the Early Indicators of Later Work Levels, Disease and Death NIH/National Institute on Aging program project grant number P01 AG10120.
Appendix A
See Tables A.1– A.5.
Table A1.
Age distribution in UA and NHANES I samples
| Age group | UA (n = 9509) (%) | NHANES I (n = 1912) (%) |
|---|---|---|
| 45–49 | 38.4 | 28.4 |
| 50–54 | 29.3 | 26.6 |
| 55–59 | 19.7 | 25.8 |
| 60–64 | 12.6 | 19.2 |
Footnotes
The prevalence of obesity is defined as a BMI of 30 kg/m2 or higher. Most of the growth in obesity happened during the last quarter of the 20th century, and seems to keep growing into the beginning of the new millennium.
The whole supplement of Public Health Nutrition 5(1A) 2002 presents the papers of the Bellagio meeting, where worldwide experts discussed the growth and prevalence of obesity in developing countries.
See Bray et al. (1998) for alternatives to measuring body fat.
NIH website revised on 18 May 2004 http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/risk.htm.
Underweight increases the risk of death even when adjusting for presence of disease and smoking history (Cornoni-Huntley et al., 1991; Calle et al., 1999).
Waaler also pointed out the inverse J-shape relationship between height and mortality.
The wives and children of deceased soldiers were also eligible for pensions.
Given the sample design of NHANES I the appropriate weights were used for all calculations.
The sub-sample of UA veterans may present a number of randomness problems due to the survival probabilities and the selection effect of undergoing a medical examination. Previous tests, however, show that those who survived and were examined had similar anthopometic characteristics to the rest of the Union Army cohort (Helmchen and Henderson, 2004).
The age distribution of the UA sample is less uniformly distributed than the NHANES I (see Table A.1 in Appendix A). As a result, UA veterans were almost a year and a half younger at examination than NHANES I subjects.
Over time, as legislation liberalized old age became sufficient to claim disability.
It is important to be cautious about the level of generalization that can be drawn from the UA sample and the level of comparability to NHANES I. In addition to the aforementioned selection problems in the UA sample, genetic and ethnic changes due to different migration patterns between the 19th and 20th century should be acknowledged, even when restricting the NHANES I sample to non-Hispanic white men. In addition, the UA was mainly a volunteer army and some of the volunteers were rejected because of health problems, whilst NHANES I is a sample drawn from the whole population. Note, however, that there is no alternative or better dataset to study BMI distributions and longevity in the 19th century US other than the UA.
For the UA both samples n = 9509 and n = 6321 are relatively similar in terms of age at measurement, BMI, and birthplace (see Tables A.2 and A.3 in Appendix A). Furthermore, for veterans who died within 20 years after the medical examination, those without information on the cause of death lived on average slightly longer (11.79 years) than those for whom we know the cause of death (11.05 years).
Relative risk in relation to the mean risk. Table A.1 in Appendix A shows the absolute risk of death within 20 years of the first examination by BMI class. The table includes the 95% confidence intervals.
The absolute mean risk f death was higher for NHANES I than for the UA. Yet, there is no statistical difference between the 95% confidence intervals.
Breaking down BMI categories into one-unit intervals provides a similar pattern, but far more erratic due to the low sample size within each interval.
The regression used to fit a cubic function is not shown in the paper, but is available upon request. Other authors have found an even higher nadir for BMI risk using quadratic fits in NHANES I (Allison et al., 2002; Su, 2003) and for HRS (Henderson, 2001).
Although the hazard ratio for men in NHANES I with a BMI of 25–30 was a little higher than those with a BMI of 20–25 the level of statistical significance is questionable. Non-Hispanic white men with a BMI between 25 and 30 have a higher hazard ratio than the all the men together, but once again the level of statistical significance is low.
The actual low risk range for NHANES I may in the 20–22 BMI range. For all men the hazard ratio (HR = 1.14) is relatively larger for this group than for the 24–24 BMI range, but the statistical significance is not very robust (P > |z| = 0.497). For white men only, however, the hazard ratio of the 20–22 MBI group is even larger (HR = 1.36) with a higher level of statistical significance (P > |z| = 0.174).
The results are very similar if the “normal” category runs from a BMI of 18.5 to 25 instead of 20–25. The main difference is that underweight men are at a higher risk when the limit is set to a BMI of 18.5 instead of 20.
The recorded cause of death may have some accuracy problems especially in the UA cohort when the methods of diagnosis were not as precise as in the late 20th century. In addition, only the first cause of death was used and in many cases people have multiple causes of death.
Alternative causes of death were treated as right censored in the competing risk model.
The term “epidemiologic transition” was first introduced by Abdel Omran (1971).
The actual causality between those who had a low BMI and died of infectious disease is hard to predict, especially for those who died rapidly after the examination. Some may have had a low BMI because they were infected while some others may have been more susceptible to infection because of their low weight.
Note that the actual hazard ratios are substantially larger than 1 for NHANES I examinees with a BMI below 20, especially for non-Hispanic white men. Yet, it is hard to draw any conclusions given the statistical significance of the estimates.
The hazard ratio for all men in NHANES I with a BMI of 30 or above was 1.716 (P > |z| = 0.050) in comparison to those in the 20–30 BMI group. The hazard ratio for non-Hispanic white men with a BMI of 30 or larger was 1.457 (P > |z| = 0.179). The regression is available upon request.
Note that being underweight for non-Hispanic white men in NHANES I may increase the risk of death from CVD since the hazard was 1.723 (P > |z| = 0.181).
The risk of death from digestive disorders for all veterans was the highest for those with a BMI between 25 and 30, an effect that did not apply to those examined after 1890.
The effect of a BMI over 35 is unclear probably due to the low proportion of deaths from endocrine, nutritional and metabolic diseases, and immunity disorders.
The regressions for causes of death included in “other” are available upon request.
The description provided by the boards of surgeons that examined the Union Army veterans provides some evidence of the adipose accumulation in some of the men with a BMI under 30. In some cases, the board of physicians described some of the general characteristics of the examinee. Surgeons described among other factors the complexion of the veterans, and in some cases made allusion to their weight and fat. Physicians were not required to describe the weight complexion of their subject, and therefore the record was not made systematically. We can find, however, a small number of references to veterans being “fat”, “obese” or with “abundance of adipose tissue”. Veterans that were described as “obese” had a significantly shorter lifespan than the rest of the population. On average they lived over 5 years less after the examination. Although the sample was very small altogether, most veterans described as “obese” had a BMI above 30, a few (24%) with a BMI between 25 and 30, and even that group died earlier than the rest.
Differences in relative leg length and frame size are such that two people may share the same amount of body fat, but have different leg sizes. Hence, the person with the shorter legs will have a higher BMI. On the other hand, two people can have the same BMI but one has a big frame and therefore low body fat, while the other has a small frame and a high body fat content (Deurenberg et al., 2002).
Adult height reflects the cumulative history of nutrition during developing years, while BMI responds to the current level of nutrition. A huge body of literature has developed over the past three decades on the changes in heights (see for example, Fogel, 1993; Komlos, 1994; Steckel and Floud, 1997). Studies in long-term trends in BMI are more scattered because the data on weight is harder to find. There is ample evidence, however, of growing BMIs in the United States, especially during the 20th century (see for example Costa and Steckel, 1997; Helmchen and Henderson, 2004).
The association between adult stature and survival has been found for past and present populations (see Alter, 2003 for a review). Some researchers, however, argue that shorter people are not unhealthier (Seckler, 1982).
Analogous results have been found across ethnicities that may be explained due to differentials in stature. In a comparative study, Gurrici et al. (1998) reported that Indonesians had more body fat at a lower BMI than Dutch Caucasians, and recommended that the cut-off points for obesity in Indonesia should be 27 kg/m2 instead of 30 kg/m2. It is worth mentioning that Indonesians were substantially shorter on average than the Dutch, one of the tallest contemporary populations. For instance Indonesian men (1.64 m) were 16 cm shorter than the Dutch (1.8 m), while Indonesian women were 14 cm shorter (1.53 and 1.67 m, respectively. The study was made with relatively low samples, and as the authors acknowledge “[t]he two study groups are difficult to compare with relation to socio-economic class, because of the different states of development in both countries.” (Gurrici et al., 1998, p. 781).
Around 3.4% of the increase in the probability of survivorship within the first 20 years can be explained by the increase in 1.5 cm between the UA and NHANES. The overall probability of death within the first 20 years declined from 0.489 (UA) to 0.396 (NHANES)—a 19% decrease. Additionally for both samples, an extra centimeter reduced the probability of death by–0.003. So a 1.5 cm increase in mean height would explain around 3.4% of the change in survival probability.
The UA height distribution is multimodal, maybe due to heaping. For example, the first mode is 1.524, which is equivalent to 5 feet.
The “fetal origins” hypothesis, advanced by Barker (1993, 1997, 1998), suggests that conditions in utero (most likely nutritional) will affect the development of chronic conditions later in life. Barker’s hypothesis is still controversial, for a review see Rasmussen (2001).
The World Health Organization considers babies born under 2.5 kg as underweight (WHO, 1995), but the measure may be different across time.
For children born in Boston Lying-In between 1884 and 1900, from 4.7 to 6.9 were underweight (Ward, 1993).
Children born in the 19th century may have been born to shorter mothers than those born a century later. Babies born to shorter women tend to have lower birth weight than those born to taller women. Lagiou et al. (2003) argue that children born from Caucasian American women tend to weight more than children born out of Chinese mothers. In both cases the height of the mother is positively related to the birth weight of babies, and American mothers are significantly taller than their Chinese counterpart. In addition, Chinese women who migrate to the United States tend to grow taller, heavier, and have higher weighting children.
Part of the growth in agricultural output went to non-food cash crops, such as cotton. Komlos (1987) argues that the relative price of food increased after 1940.
As a proxy of adiposity, BMI may not reflect some of the changes in fat accumulation over time resulting from changes in the structure of labor and a transition toward less physically demanding jobs.
As a result of all these transformations, life expectancy for men aged 50 increased by more than 17% from 20.54 to 24.11 years from 1900 to 1975, respectively. (Bell et al., 1992; Human Mortality Database, 2005). The life expectancy of UA veterans may have been somewhat larger than the rest of the population. On average men aged 50 in 1900 lived 22.75 years more. In addition, due to many of these improvements the disease pattern and the causes of death have changed substantially. The transformation of the disease pattern makes the comparison across ethnicities complicated. As disease patterns change over time, we may only be able to infer that diseases vary across ethnicities at a particular point in time.
References
- Allebeck P, Bergh C. Height, body mass index and mortality: do social factors explain the association? Public Health. 1992;106:375–382. doi: 10.1016/s0033-3506(05)80186-6. [DOI] [PubMed] [Google Scholar]
- Allison DB, Faith MS. On estimating the minima of BMI-mortality curves. Int J Obes Relat Metab Disord. 1996;20:496–498. [PubMed] [Google Scholar]
- Allison DB, Zhu SK, Plankey M, Faith MS, Heo M. Differential association of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes. 2002;26:410–416. doi: 10.1038/sj.ijo.0801925. [DOI] [PubMed] [Google Scholar]
- Alter, G. 2003. Stature, survival, and the standard of living: a model of the effects of diet and disease on declining mortality and increasing stature. Unpublished working paper. Indiana University, Bloomington.
- Barker DJP. The intrauterine origins of cardiovascular disease. Acta Paediatr Scand. 1993;391:93–99. doi: 10.1111/j.1651-2227.1993.tb12938.x. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Barker, D.J.P., 1998. Mothers, Babies and Health in Later Life. Churchill Livingstone, Edinburgh, Scotland.
- Bell, F.C., Wade, A.H., Goss, S.C., Life Tables for the United States Social Security Area. Actuarial Study No. 107, SSA Pub. No. 11-11536, 1992.
- Bray, G.A., Bouchard, C., James, W.P.T., 1998. Definitions and proposed current classification of obesity. In: Bray, G.A., Bouchard, C., James, W.P.T. (Eds.), Handbook of Obesity. M. Decker, New York, pp. 31–40.
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C. Body mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of US Adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control, 2004. www.cdc.gov, revised on 1 August 2004.
- Cornoni-Huntley JC, Harris TB, Everett DF, Albanes D, Micozzi MS, Miles TP, Feldman JJ. An overview of body weight of older persons, including the impact on mortality. The National Health and Nutrition Examination Survey I–Epidemiologic Follow-up Study. J Clin Epidemiol. 1991;44:743–753. doi: 10.1016/0895-4356(91)90126-t. [DOI] [PubMed] [Google Scholar]
- Costa DL. Height, weight, wartime stress, and older age mortality: evidence from the Union Army records. Explor Econ Hist. 1993;30:424–449. [Google Scholar]
- Costa DL. Unequal at birth: a long-term comparison of income and birth weight. J Econ Hist. 1998;58:987–1009. [Google Scholar]
- Costa DL. The measure of man and older age mortality: evidence from the Gould sample. J Econ Hist. 2004;64:1–23. [Google Scholar]
- Costa, D.L., Steckel, R.H., 1997. Long-term trends in health, welfare, and economic growth in the United States. In: Steckel, R.H., Floud, R. (Eds.), Health and Welfare during Industrialization. University of Chicago Press, Chicago.
- Deurenberg P, Weststrate JA, Seidell JC. Body Mass Index as a measure of body fatness: age and sex specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Deurenberg-Yap M, Gurricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- Engeland A, Tretli S, Bjørge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RW. New sources and new techniques for the study of secular trends in the study of nutritional status, health, mortality, and the process of aging. Hist Methods. 1993;26:5–43. [Google Scholar]
- Fogel RW. Economic growth, population theory and physiology: the bearing of long-term processes on the making of economic policy. Am Econ Rev. 1994;84:369–395. [Google Scholar]
- Fogel, R.W., 1999. Public Use Tape on the Aging of the Veterans of the Union Army: Surgeon’s Certificates, 1860–1940, Version S-1 Unstandardized (Center for Population Economics, University of Chicago, Graduate School of Business, and Department of Economics, Brigham Young University).
- Fogel, R.W., 2000a. Public Use Tape on the Aging of the Veterans of the Union Army: Military, Pension, and Medical Records, 1860–1940, Version M-5 (Center for Population Economics, University of Chicago, Graduate School of Business, and Department of Economics, Brigham Young University).
- Fogel, R.W., 2000b. The Fourth Great Awakening and the Future of Egalitarianism. University of Chicago Press, Chicago.
- Fogel, R.W., 2001. Early indicators of Later Work Levels, Disease, and Death. Unpublished typescript. Center for Population Economics, University of Chicago.
- Fogel, R.W., 2004. The Escape from Hunger and Premature Death. University of Chicago Press, Chicago, 1700–2100.
- Fogel, R.W. and Canavese P., 2004. Arthritis: Changes in Its Prevalence during the 19th and 20th Centuries. Unpublished working paper, Center for Population Economics, University of Chicago.
- Gallager D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is BMI for comparison of body fatness across age, se, and ethnic groups. Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- Garrow, J.S., 1981. Treat Obesity Seriously: A Clinical Manual. Churchill Livingstone, London.
- Garrow JS, Webster J. Quetelet’s index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–153. [PubMed] [Google Scholar]
- Glasson, W.H., 1918. Federal Military Pensions in the United Sates. Oxford University Press, New York.
- Goldin C, Margo RA. The Poor at Birth: Weights and Infant Mortality at Philadelphia’s Almshouse Hospital, 1848–1873. Explor Econ Hist. 1989;69:360–379. [Google Scholar]
- Gordon T, Kannel WB. Obesity and cardiovascular diseases: the Framinham study. Clin Endocrinol Metab. 1976;5:367–375. doi: 10.1016/s0300-595x(76)80026-6. [DOI] [PubMed] [Google Scholar]
- Gurrici S, Hartiyanti Y, Hautvast JGAJ, Deurenberg P. Relationship between body fat and body mass index: differences between Indonesians and Dutch Caucasians. Eur J Clin Nutr. 1998;52:779–783. doi: 10.1038/sj.ejcn.1600637. [DOI] [PubMed] [Google Scholar]
- Haines, M.R., 1998. Health, Height, Nutrition, and Mortality: Evidence on the “Antebellum Puzzle” from Union Army Recruits in the Middle of the Nineteenth Century. NBER Historical paper 107.
- Haines, M.R., Craig, L.A., Weiss T., 2000. Development, health, nutrition, and mortality: the case of the “Antebellum Puzzle” in the United States. NBER Historical Paper 130.
- Health and Welfare Canada, 1988. Canadian Guidelines for Healthy Weights. Report of an Expert Group Convened by Health Promotion Directorate, Health Services and Promotion Branch (Health and Welfare Canada, Ottawa).
- Health and Welfare Canada, 2003. Canadian Guidelines for Body Weight Classification in Adults. Office of Nutrition Policy and Promotion (Health and Welfare Canada, Otawa).
- Helmchen LA, Henderson RM. Changes in the distribution of body mass index of white US men. Ann Hum Biol. 2004;31:174–181. doi: 10.1080/03014460410001663434. 1890–2000. [DOI] [PubMed] [Google Scholar]
- Henderson, R. M., 2001. Predictors of mortality for older Americans during the 20th century #2001-5/CPE Working Paper Series, Center for Population Economics, University of Chicago.
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an Independent Risk Factor for Cardiovascular Disease: a 26-Year Follow-up of Participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- Human Mortality Database. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Available at www.mortality.org or www.humanmortality.de (data downloaded on 3-5-2005).
- International Obesity Task Force, International Association for the Study of Obesity, 2000. The Asia-Pacific Perspective: Redefining Obesity and its Treatment (WHO Western Pacific Region).
- James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obesity Res. 2001;9 (suppl 4):228S–233S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Hedblad B, Engtstrom G, Nilsson P, Berglund G, Janzon I. Influence of Obesity on Cardiovascular Risk. Twenty-three-year follow-up of 22 025 men from an urban Swedish. Population. 2002;26:1046–1053. doi: 10.1038/sj.ijo.0802060. [DOI] [PubMed] [Google Scholar]
- Kim, John M. 1996. The Economics of Nutrition, Body Build, and Health: Waaler Surfaces and Physical Human Capital. Ph.D. dissertation, University of Chicago.
- Komlos J. The height and weight of West Point cadets: Dietary Change in Antebellum America. J Econ Hist. 1987;47:897–927. [Google Scholar]
- Komlos, J. (Ed.),.1994. Stature, Living Standards, and Economic Development: Essays in Anthropometric History. University of Chicago Press, Chicago.
- Komlos J. Anomalies in Economic History: Toward a Resolution of the “Antebellum Puzzle”. J Econ Hist. 1996;56:202–214. [Google Scholar]
- Komlos J, Baur M. From the tallest to (one of) the fattest: the enigmatic fate of the American population in the 20th century. Econ Hum Biol. 2004;2:57–74. doi: 10.1016/j.ehb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Hsieh CC, Trichopoulos D, Xu B, Wuu J, Mucci L, Tamimi R, Adami HO, Cnattingius S. Birthweight differences between USA and China and their relevance to breast cancer Aetiology. Int J Epidemiol. 2003;32:193–198. doi: 10.1093/ije/dyg047. [DOI] [PubMed] [Google Scholar]
- Lee, I.M., Manson, J.E., Henneckens, C.H., Paffenbarger R.S. Jr., 1993. Body Weight and Mortality. [DOI] [PubMed]
- National Center For Health Statistics [NCHS], 1973. Plan and Operation of the Health and Nutrition Examination Survey, United States 1971–1973. Vital Health Statistics, Series 1, vol. 10. [PubMed]
- National Center for Health Statistics [NCHS], 1992. Plan and Operation of the NHANES I Epidemiology Followup Study, 1987. Vital Health Statistics Series 1, vol. 27, DHHS publication no. (PHS) 92-1303 National Center for Health Statistics.
- National Health and Medical Research Council, 1997. Acting on Australia’s Weight: A Strategic Plan for the Prevention of Overweight and Obesity. Summary Report (Australian Government Publishing Service).
- National Institutes of Health. Consensus Development Panel on the Health Implications of Obesity. Health implications of obesity. Annals of Internal Medicine. 1985;103:1073–1077. [PubMed] [Google Scholar]
- National Institutes of Health, 2000. The Practical Guide Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. NIH publication number 00-4084.
- National Institutes of Health, 2004. www.nih.gov, revised on 1 August 2004.
- National Research Council, 1989. Recommended Dietary Allowances, 10th ed., (National Academy of Sciences).
- Norgan NG, Ferro-Luzzy A. Weight-height indices as estimators of fatness in men. Hum Nutr Clin Nutr. 1985;36c:363–372. [PubMed] [Google Scholar]
- Omran A. The epidemiologic transition: a theory of epidemiology of population change. Milbank Memorial Fund Quart. 1971;49:501–538. [PubMed] [Google Scholar]
- Popkin BM. An overview on the nutrition transition and its health implications: the Bellagio meeting. Public Health Nutr. 2002;5:93–103. doi: 10.1079/phn2001280. [DOI] [PubMed] [Google Scholar]
- Sandberg, L.G., Steckel, R.H., 1997. Was industrialization hazardous to your health? Not in Sweden!. In: Steckel, R.H., Floud, R. (Eds.), Health and Welfare During Industrialization. University of Chicago Press, Chicago.
- Seckler, D, 1982. Small but healthy: A basic Hypothesis in the Theory, Measurement and Policy of Malnutrition. In: Sukhatme, P.V., (Ed.), Newer Concepts in Nutrition and Their Implications for Policy (Maharastra Association for the Cultivation of Sciences, Pune, India).
- Secretaría de Salubridad y Asistencia, 1998. Norma Oficial Mexicana NOM-174-SSA1-1998, Para el Manejo Integral de la Obesidad (SSA, Mexico).
- Song YM, Smith GD, Sung J. Adult Height and Cause-specific Mortality: A Large Prospective Study of South Korean Men. Am J Epidemiol. 2003;158:479–485. doi: 10.1093/aje/kwg173. [DOI] [PubMed] [Google Scholar]
- Steckel, R.H., 1992. Stature and Living Standards in the United States. In: Gallman, R.E., Wallis, J.J. (Eds.), Economic Growth and Standards of Living before the Civil War. University of Chicago Press, Chicago, pp. 265–308.
- Steckel RH. Stature and the standard of living. J Econ Lit. 1995;33:1903–1940. [Google Scholar]
- Strandberg TE. Inverse relation between height and cardiovascular mortality in men during 30-year follow-up. Am J Cardiol. 1997;80 (3):349–350. doi: 10.1016/s0002-9149(97)00362-7. [DOI] [PubMed] [Google Scholar]
- Su, Dejun 2003. Body Mass Index and Old Age Survival: A Comparative Study Between the Union Army Records and the NHANES-I Epidemiological Follow-up Sample1. Typescript, Center for Population Economics, University of Chicago, 2004. [DOI] [PubMed]
- Susser M, Stein Z. Timing in prenatal nutrition: a reprise of the Dutch Famine Study. Nutr Rev. 1994;52:84–94. doi: 10.1111/j.1753-4887.1994.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Frongillo EA, Jr, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord. 1996;20:63–75. [PubMed] [Google Scholar]
- Tuomilehto J, Salonen JT, Marti B, Jalkanen L, Puska P, Nissinen A, Wolf E. Body Weight and Risk of Myocardial Infraction and Death in the Adult Population of Eastern Finland. Br J Med (Clinical Research Edition) 1987;295:623–627. doi: 10.1136/bmj.295.6599.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Bureau of Pensions, 1925, Laws of the United States Governing the Granting of Union Army and Navy Pensions (Government Printing Office, Washington).
- USDA/USDHH, 2000. Dietary Guidelines for Americans, 5th ed. Home and Garden Bulletin 232.
- Visscher TL, Seidell JC, Menotti A, Blackburn H, Nissinen A, Feskens EJ. Underweight and overweight in relation to mortality among men aged 40–59 and 50–69 years: the Seven Countries Study. Am J Epidemiol. 2000;151:660–666. doi: 10.1093/oxfordjournals.aje.a010260. [DOI] [PubMed] [Google Scholar]
- Waaler HT. Height, weight, and mortality: the Norwegian experience. Acta Medica Scand suppl. 1984;679:1–51. doi: 10.1111/j.0954-6820.1984.tb12901.x. [DOI] [PubMed] [Google Scholar]
- Ward, W.P., 1993. Birth Weigh and Economic Growth: Women’s Living Standards in the Industrializing West. The University of Chicago Press, Chicago.
- Womersley J, Durnin JVGA. A comparison of the skinfold method with extent overweight and various weight-height relationships in the assessment of obesity. Br J Nutr. 1977;38:271–284. doi: 10.1079/bjn19770088. [DOI] [PubMed] [Google Scholar]
- World Health Org. Expert Comm. Phys. Status, 1995. Physical Status: The Use and Interpretation of Anthropometry. WHO Tech. Rep. Ser. No. 854 (World Health Organization, Geneva, Switzerland). [PubMed]
- World Health Organization, 1998. Obesity: Preventing and Managing the Global Epidemic. (World Health Organization, Geneva). [PubMed]
- World Health Organization. 2000. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. (Health Communications Australia, Australia).
- Yajnik CS. Obesity epidemic in India: intrauterine origins? Proc Nutr Soc. 2004;63 (3):387–396. doi: 10.1079/pns2004365. [DOI] [PubMed] [Google Scholar]


