Abstract
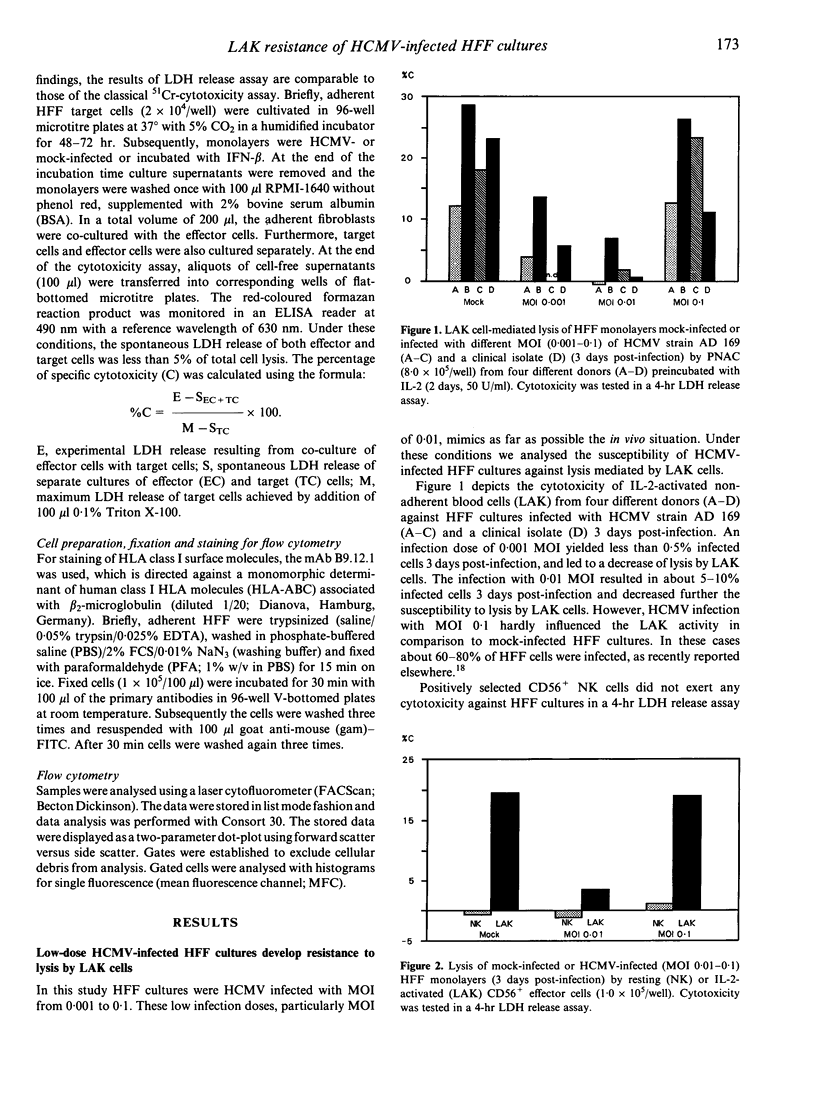
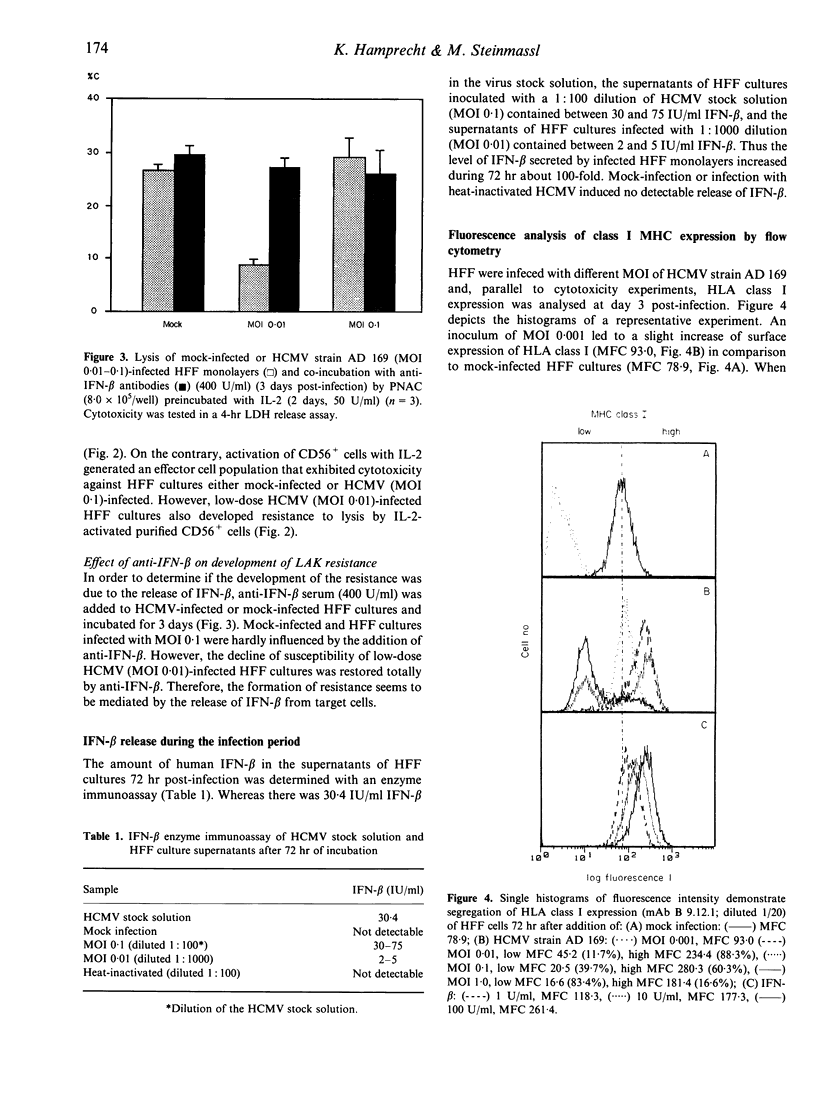
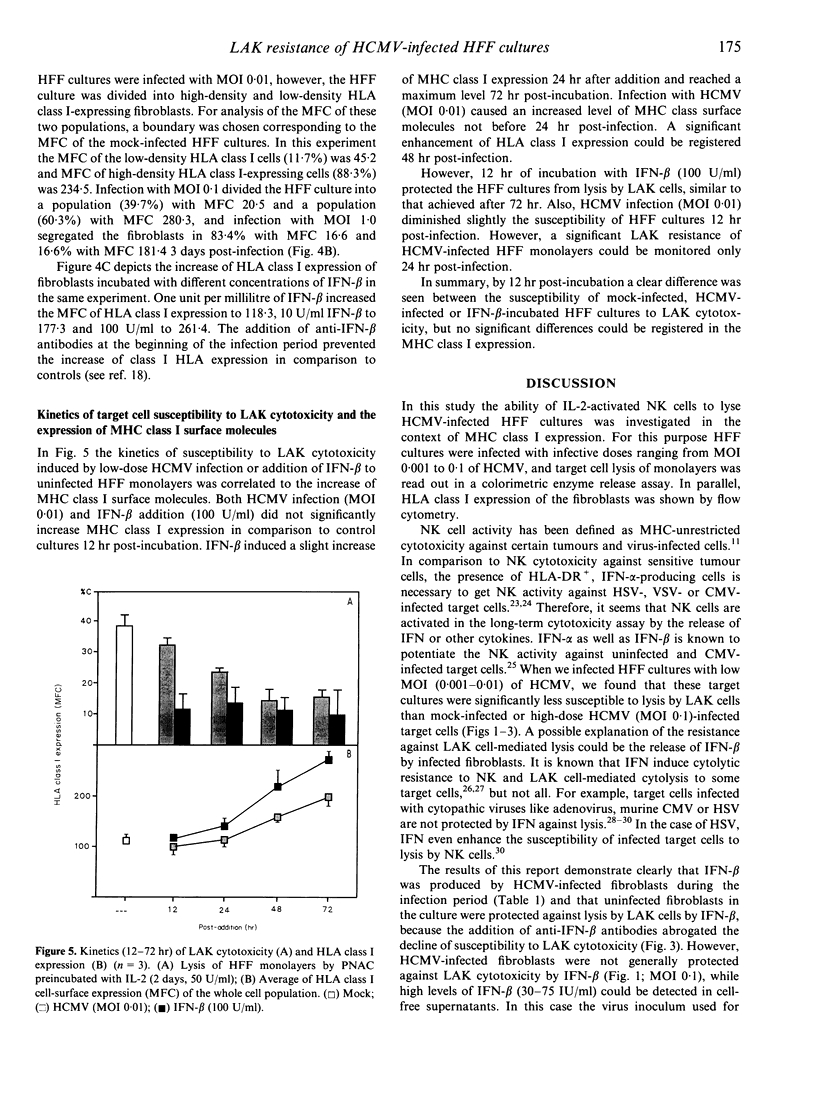
We have investigated the susceptibility of human foreskin fibroblast (HFF) monolayers infected at low level with human cytomegalovirus (HCMV) strain AD 169, or a clinical HCMV isolate, to lysis mediated by interleukin-2(IL-2)-activated killer cells (LAK). HFF cultures inoculated with a multiplicity of infection (MOI) dose of 0.001-0.01 had significantly decreased susceptibility to lysis by IL-2-activated non-adherent blood cells (PNAC) or purified CD56+ cells in comparison to mock-infected cultures. By 12h post incubation HFF cultures showed diminished susceptibility to LAK cell cytotoxicity when HFF cultures were incubated with HCMV (MOI 0.01) or interferon-beta (IFN-beta; 100 U/ml). Cytofluorometric analysis of HCMV-infected HFF cultures showed a modulation of HLA class I expression on single cells 3 days post-infection, namely, segregation of the cells in low- and high-density HLA class I-expressing cells depended on the dose of HCMV inoculum. However, up-regulation of HLA class I surface molecules was not significantly enhanced 12 h post-incubation with HCMV inoculum or IFN-beta. Anti-IFN-beta antibodies prevented both the development of the resistance and the increase of HLA class I expression in infected HFF cultures. In summary, the comparison of HLA class I expression and the LAK susceptibility of HCMV-infected HFF cultures may lead to the following conclusions: IFN-beta mediates the protection of neighbouring uninfected fibroblasts, but the modulation of HLA class I expression on uninfected cells does not correlate with the diminished susceptibility.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay S., Miller D. S., Matsumoto-Kobayashi M., Clark S. C., Starr S. E. Effects of interferons and interleukin 2 on natural killing of cytomegalovirus-infected fibroblasts. Clin Exp Immunol. 1987 Feb;67(2):372–382. [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S., Perussia B., Trinchieri G., Miller D. S., Starr S. E. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J Exp Med. 1986 Jul 1;164(1):180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks T. A., Rouse B. T. Herpesviruses--immune escape artists? Clin Infect Dis. 1992 Apr;14(4):933–941. doi: 10.1093/clinids/14.4.933. [DOI] [PubMed] [Google Scholar]
- Barnes P. D., Grundy J. E. Down-regulation of the class I HLA heterodimer and beta 2-microglobulin on the surface of cells infected with cytomegalovirus. J Gen Virol. 1992 Sep;73(Pt 9):2395–2403. doi: 10.1099/0022-1317-73-9-2395. [DOI] [PubMed] [Google Scholar]
- Beck S., Barrell B. G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988 Jan 21;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- Biron C. A., Byron K. S., Sullivan J. L. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989 Jun 29;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Browne H., Smith G., Beck S., Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature. 1990 Oct 25;347(6295):770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- Brutkiewicz R. R., Klaus S. J., Welsh R. M. Window of vulnerability of vaccinia virus-infected cells to natural killer (NK) cell-mediated cytolysis correlates with enhanced NK cell triggering and is concomitant with a decrease in H-2 class I antigen expression. Nat Immun. 1992 Jul-Aug;11(4):203–214. [PubMed] [Google Scholar]
- Bukowski J. F., Welsh R. M. Inability of interferon to protect virus-infected cells against lysis by natural killer (NK) cells correlates with NK cell-mediated antiviral effects in vivo. J Immunol. 1985 Nov;135(5):3537–3541. [PubMed] [Google Scholar]
- Chadwick B. S., Miller R. G. Hybrid resistance in vitro. Possible role of both class I MHC and self peptides in determining the level of target cell sensitivity. J Immunol. 1992 Apr 1;148(7):2307–2313. [PubMed] [Google Scholar]
- Fujinami R. S., Nelson J. A., Walker L., Oldstone M. B. Sequence homology and immunologic cross-reactivity of human cytomegalovirus with HLA-DR beta chain: a means for graft rejection and immunosuppression. J Virol. 1988 Jan;62(1):100–105. doi: 10.1128/jvi.62.1.100-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht K., Vötsch W., Anderer F. A. Activation of human monocyte and natural killer cell-mediated tumour cell killing by two dialysable thymic factors. Scand J Immunol. 1986 Jul;24(1):59–71. doi: 10.1111/j.1365-3083.1986.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Howell D. M., Fitzgerald-Bocarsly P. Natural killer-mediated lysis of some but not all HSV-1- or VSV-infected targets requires the participation of HLA-DR-positive accessory cells. Immunology. 1991 Mar;72(3):443–447. [PMC free article] [PubMed] [Google Scholar]
- Ibanez C. E., Schrier R., Ghazal P., Wiley C., Nelson J. A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991 Dec;65(12):6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. S., Schoon R. A., Leibson P. J. MHC class I expression on tumor targets inhibits natural killer cell-mediated cytotoxicity without interfering with target recognition. J Immunol. 1993 Feb 15;150(4):1429–1436. [PubMed] [Google Scholar]
- Kaufman D. S., Schoon R. A., Leibson P. J. Role for major histocompatibility complex class I in regulating natural killer cell-mediated killing of virus-infected cells. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8337–8341. doi: 10.1073/pnas.89.17.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski C., Callewaert D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983 Nov 25;64(3):313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Lin Y., Proud G., Taylor R. M., Kirby J. A. Renal allograft rejection: protection of renal epithelium from natural killer cells by cytokine-induced up-regulation of class I major histocompatibility antigens. Immunology. 1993 Jun;79(2):290–297. [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Experimental strategies and interpretations in the analysis of changes in MHC gene expression during tumour progression. Opposing influences of T cell and natural killer mediated resistance? J Immunogenet. 1986 Apr-Jun;13(2-3):141–151. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Lobo P. I., Spencer C. E. Use of anti-HLA antibodies to mask major histocompatibility complex gene products on tumor cells can enhance susceptibility of these cells to lysis by natural killer cells. J Clin Invest. 1989 Jan;83(1):278–287. doi: 10.1172/JCI113870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnati M. S., Lusso P., Ciccone E., Moretta A., Moretta L., Long E. O. Recognition of virus-infected cells by natural killer cell clones is controlled by polymorphic target cell elements. J Exp Med. 1993 Sep 1;178(3):961–969. doi: 10.1084/jem.178.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley D. J., Pound J. D. Modulation of MHC antigen expression by viruses and oncogenes. Immunol Today. 1991 Dec;12(12):429–431. doi: 10.1016/0167-5699(91)90013-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating J. A., Griffiths P. D., Grundy J. E. Cytomegalovirus in urine specimens has host beta 2 microglobulin bound to the viral envelope: a mechanism of evading the host immune response? J Gen Virol. 1987 Mar;68(Pt 3):785–792. doi: 10.1099/0022-1317-68-3-785. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., Griffiths P. D., Weiss R. A. HIV susceptibility conferred to human fibroblasts by cytomegalovirus-induced Fc receptor. Nature. 1990 Feb 15;343(6259):659–661. doi: 10.1038/343659a0. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Carrasco L., Fresno M. Enhancement of susceptibility of HSV-1-infected cells to natural killer lysis by interferon. J Immunol. 1983 Aug;131(2):783–787. [PubMed] [Google Scholar]
- Perussia B. Lymphokine-activated killer cells, natural killer cells and cytokines. Curr Opin Immunol. 1991 Feb;3(1):49–55. doi: 10.1016/0952-7915(91)90076-d. [DOI] [PubMed] [Google Scholar]
- Reiter Z. Dual effects of cytokines in regulation of MHC-unrestricted cell mediated cytotoxicity. Crit Rev Immunol. 1993;13(1):1–34. [PubMed] [Google Scholar]
- Reiter Z., Fischer D. G., Rubinstein M. The protective effect of interferon against natural killing activity is not mediated via the expression of class I MHC antigens. Immunol Lett. 1988 Apr;17(4):323–328. doi: 10.1016/0165-2478(88)90005-3. [DOI] [PubMed] [Google Scholar]
- Routes J. M. Adenovirus E1A inhibits IFN-induced resistance to cytolysis by natural killer cells. J Immunol. 1993 May 15;150(10):4315–4322. [PubMed] [Google Scholar]
- Steinmassl M., Hamprecht K. Double fluorescence analysis of human cytomegalovirus (HCMV) infected human fibroblast cultures by flow cytometry: increase of class I MHC expression on uninfected cells and decrease on infected cells. Arch Virol. 1994;135(1-2):75–87. doi: 10.1007/BF01309766. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Alexander J., Payne J. A., Dawson J. R., Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkus W. J., Dawson J. R. Target structures involved in natural killing (NK): characteristics, distribution, and candidate molecules. Crit Rev Immunol. 1991;10(5):393–416. [PubMed] [Google Scholar]
- Storkus W. J., Salter R. D., Cresswell P., Dawson J. R. Peptide-induced modulation of target cell sensitivity to natural killing. J Immunol. 1992 Aug 15;149(4):1185–1190. [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Granato D., Perussia B. Interferon-induced resistance of fibroblasts to cytolysis mediated by natural killer cells: specificity and mechanism. J Immunol. 1981 Jan;126(1):335–340. [PubMed] [Google Scholar]
- Welsh R. M., Karre K., Hansson M., Kunkel L. A., Kiessling R. W. Interferon-mediated protection of normal and tumor target cells against lysis by mouse natural killer cells. J Immunol. 1981 Jan;126(1):219–225. [PubMed] [Google Scholar]
- Yamashita Y., Shimokata K., Mizuno S., Yamaguchi H., Nishiyama Y. Down-regulation of the surface expression of class I MHC antigens by human cytomegalovirus. Virology. 1993 Apr;193(2):727–736. doi: 10.1006/viro.1993.1181. [DOI] [PubMed] [Google Scholar]