Abstract
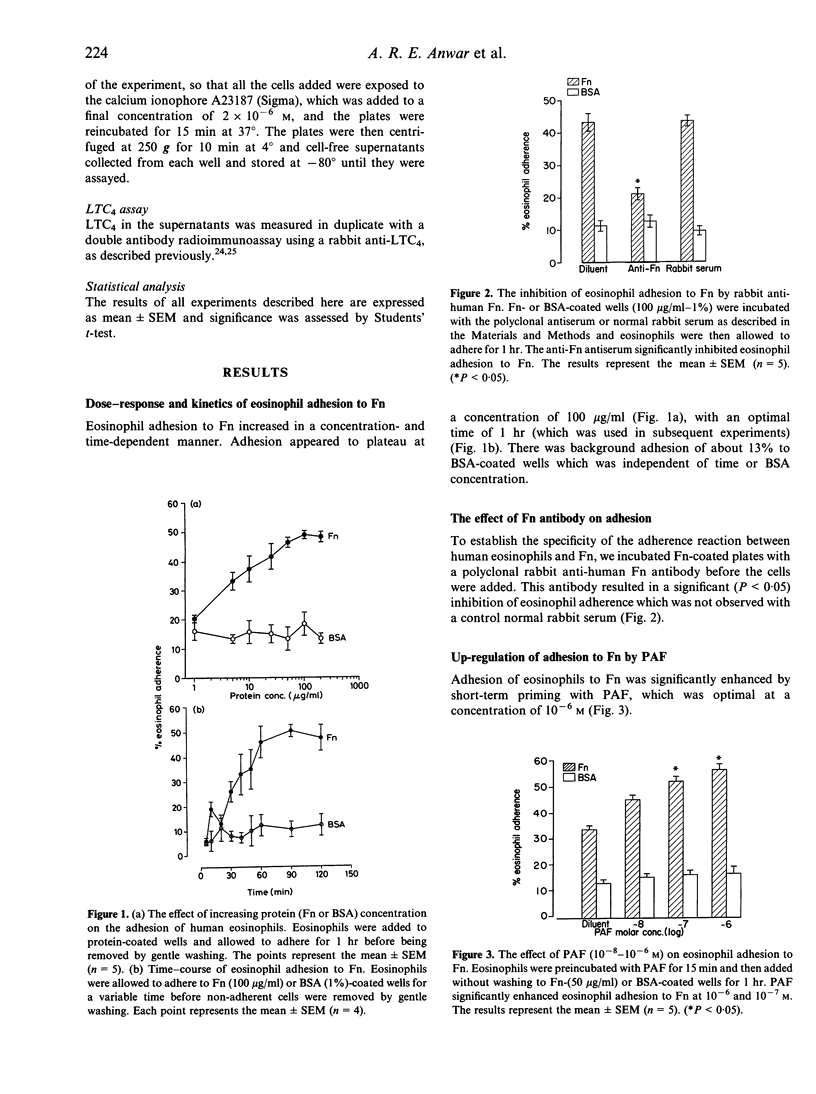
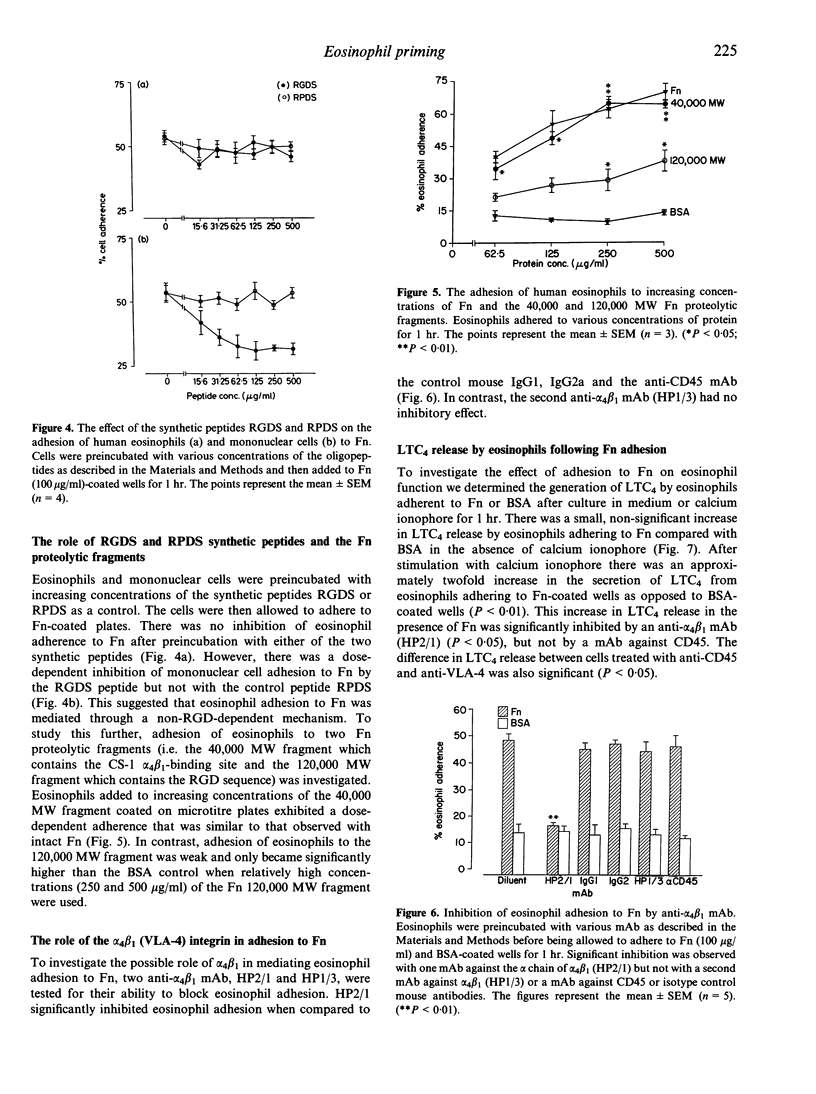
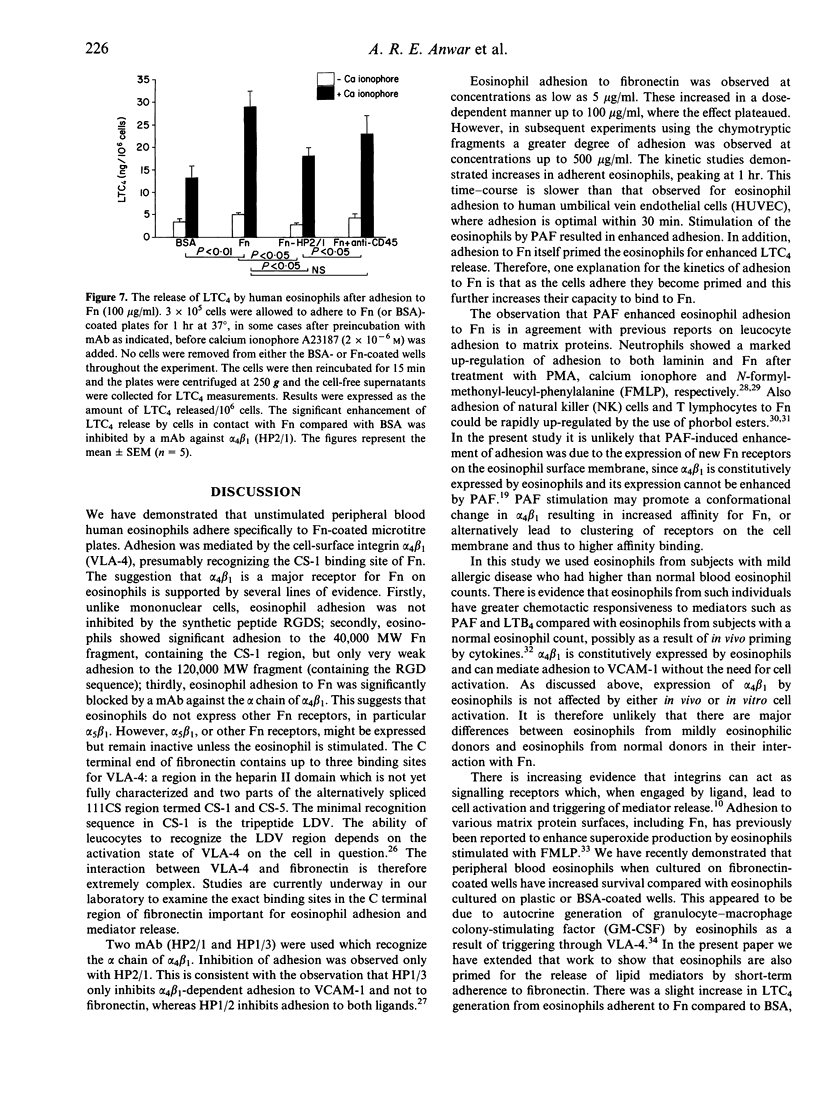
Human peripheral blood eosinophils adhered specifically to microtitre plates coated with plasma fibronectin (Fn) in a dose- and time-dependent fashion. Adhesion was optimal at 60 min at a concentration of 100 micrograms/ml. Adherence to Fn was up-regulated by platelet-activating factor (PAF; optimum concentration of 10(-6) M) and was significantly inhibited by a polyclonal anti-Fn antibody (P < 0.05). The following evidence suggested that eosinophil adhesion to Fn was mediated by alpha 4 beta 1: (1) eosinophil adherence to Fn was not inhibited by an Arg-Gly-Asp-Ser (RGDS) synthetic peptide; (2) there was a dose-dependent adherence of eosinophils to microtitre plates coated with the 40,000 MW proteolytic fragment of Fn that contains the CS-1 alpha 4 beta 1 binding region, whereas adherence to the 120,000 MW chymotryptic fragment of Fn, which contains the RGD-dependent binding site, was weak and only observed at high concentrations (> 250 micrograms/ml); (3) significant inhibition of eosinophil adherence to Fn was achieved by monoclonal antibodies (mAb) against the alpha chain of VLA-4 but not by a mAb against CD45 or a mouse myeloma antibody as negative controls. After adhesion to Fn, eosinophils were investigated for their capacity to release leukotriene C4 in response to stimulation with a suboptimal concentration of calcium ionophore (2 x 10(-6) M). Significant enhancement of release was detected with Fn-coated plates but not with the control bovine serum albumin (BSA) (P < 0.01). Furthermore, this enhancement was significantly inhibited by the alpha 4 beta 1 mAb HP2/1 (P < 0.05) but not by an anti-CD45 mAb. From these studies we conclude that (1) alpha 4 beta 1 (VLA-4) integrin is a major receptor for Fn on human eosinophils and (2) adhesion to Fn may prime eosinophils for mediator release during allergic inflammation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar A. R., Moqbel R., Walsh G. M., Kay A. B., Wardlaw A. J. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993 Mar 1;177(3):839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991 Jun 1;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack J. F., Akiyama S. K., Damsky C. H., Knape W. A., Zimmerman G. A. Human neutrophil adherence to laminin in vitro. Evidence for a distinct neutrophil integrin receptor for laminin. J Exp Med. 1990 Apr 1;171(4):1221–1237. doi: 10.1084/jem.171.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell O., Shaw R. J., Walsh G. M., Mallet A. I., Kay A. B. Inhibition of leukotriene C4 and B4 generation by human eosinophils and neutrophils with the lipoxygenase pathway inhibitors U60257 and BW755C. Int J Immunopharmacol. 1985;7(5):775–781. doi: 10.1016/0192-0561(85)90165-1. [DOI] [PubMed] [Google Scholar]
- Dobrina A., Menegazzi R., Carlos T. M., Nardon E., Cramer R., Zacchi T., Harlan J. M., Patriarca P. Mechanisms of eosinophil adherence to cultured vascular endothelial cells. Eosinophils bind to the cytokine-induced ligand vascular cell adhesion molecule-1 via the very late activation antigen-4 integrin receptor. J Clin Invest. 1991 Jul;88(1):20–26. doi: 10.1172/JCI115278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dri P., Cramer R., Spessotto P., Romano M., Patriarca P. Eosinophil activation on biologic surfaces. Production of O2- in response to physiologic soluble stimuli is differentially modulated by extracellular matrix components and endothelial cells. J Immunol. 1991 Jul 15;147(2):613–620. [PubMed] [Google Scholar]
- Dufour S., Duband J. L., Humphries M. J., Obara M., Yamada K. M., Thiery J. P. Attachment, spreading and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J. 1988 Sep;7(9):2661–2671. doi: 10.1002/j.1460-2075.1988.tb03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Hemler M. E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Gismondi A., Morrone S., Humphries M. J., Piccoli M., Frati L., Santoni A. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol. 1991 Jan 1;146(1):384–392. [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Hayes E. C., Lombardo D. L., Girard Y., Maycock A. L., Rokach J., Rosenthal A. S., Young R. N., Egan R. W., Zweerink H. J. Measuring leukotrienes of slow reacting substance of anaphylaxis: development of a specific radioimmunoassay. J Immunol. 1983 Jul;131(1):429–433. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Wheldon L. A., Komoriya A., Wayner E. A., Yamada K. M., Humphries M. J. Affinity chromatographic isolation of the melanoma adhesion receptor for the IIICS region of fibronectin and its identification as the integrin alpha 4 beta 1. J Biol Chem. 1990 Mar 5;265(7):4020–4024. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Schwartz B. R., Wayner E. A., Carlos T. M., Ochs H. D., Harlan J. M. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990 Jun;85(6):2019–2022. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehmi R., Wardlaw A. J., Cromwell O., Kurihara K., Waltmann P., Kay A. B. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992 Jun 1;79(11):2952–2959. [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Suchard S. J., Burton M. J., Dixit V. M., Boxer L. A. Human neutrophil adherence to thrombospondin occurs through a CD11/CD18-independent mechanism. J Immunol. 1991 Jun 1;146(11):3945–3952. [PubMed] [Google Scholar]
- Takada Y., Huang C., Hemler M. E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987 Apr 9;326(6113):607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Walsh G. M., Mermod J. J., Hartnell A., Kay A. B., Wardlaw A. J. Human eosinophil, but not neutrophil, adherence to IL-1-stimulated human umbilical vascular endothelial cells is alpha 4 beta 1 (very late antigen-4) dependent. J Immunol. 1991 May 15;146(10):3419–3423. [PubMed] [Google Scholar]
- Wardlaw A. J., Kay A. B. The role of the eosinophil in the pathogenesis of asthma. Allergy. 1987 Jul;42(5):321–335. doi: 10.1111/j.1398-9995.1987.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Kovach N. L. Activation-dependent recognition by hematopoietic cells of the LDV sequence in the V region of fibronectin. J Cell Biol. 1992 Jan;116(2):489–497. doi: 10.1083/jcb.116.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]


