Abstract
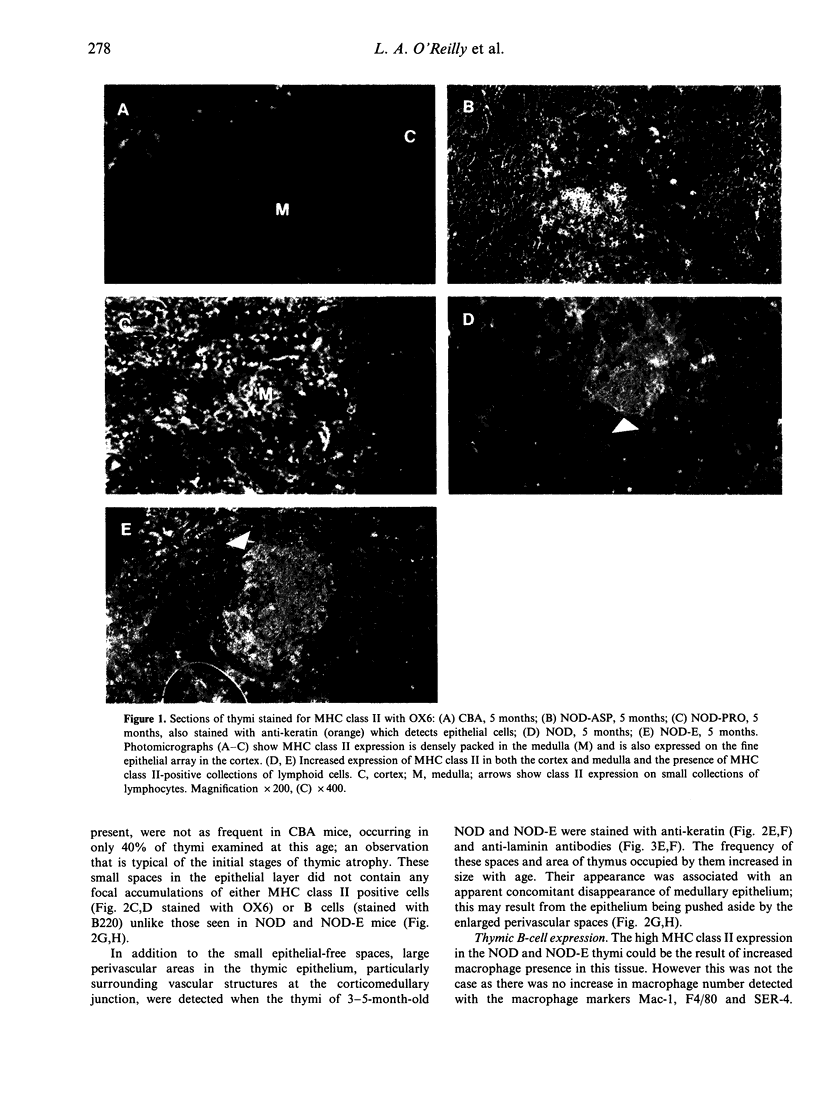
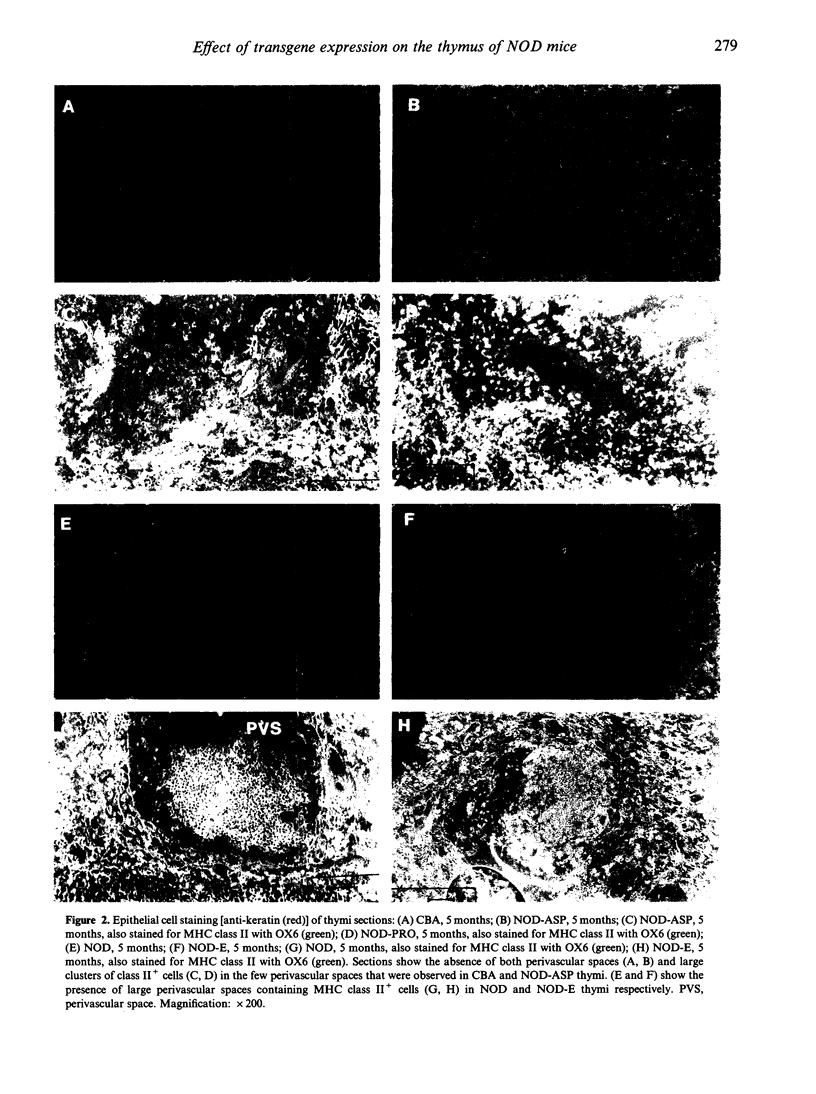
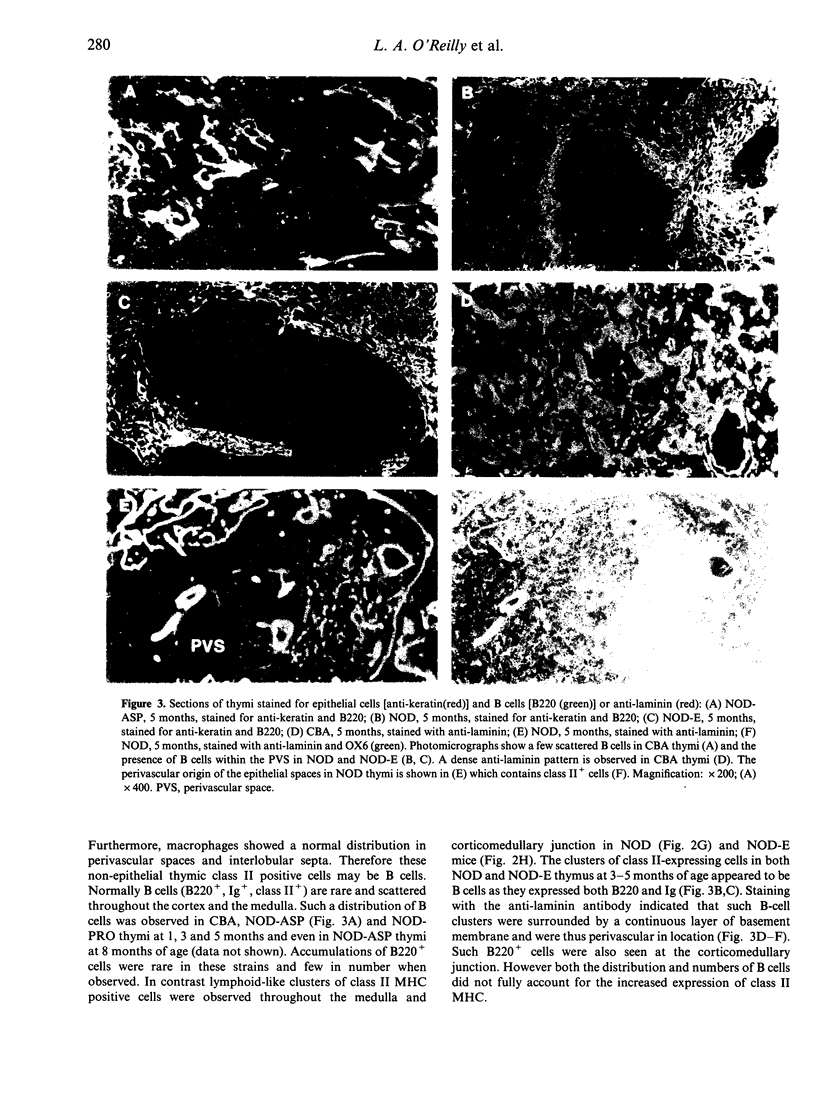
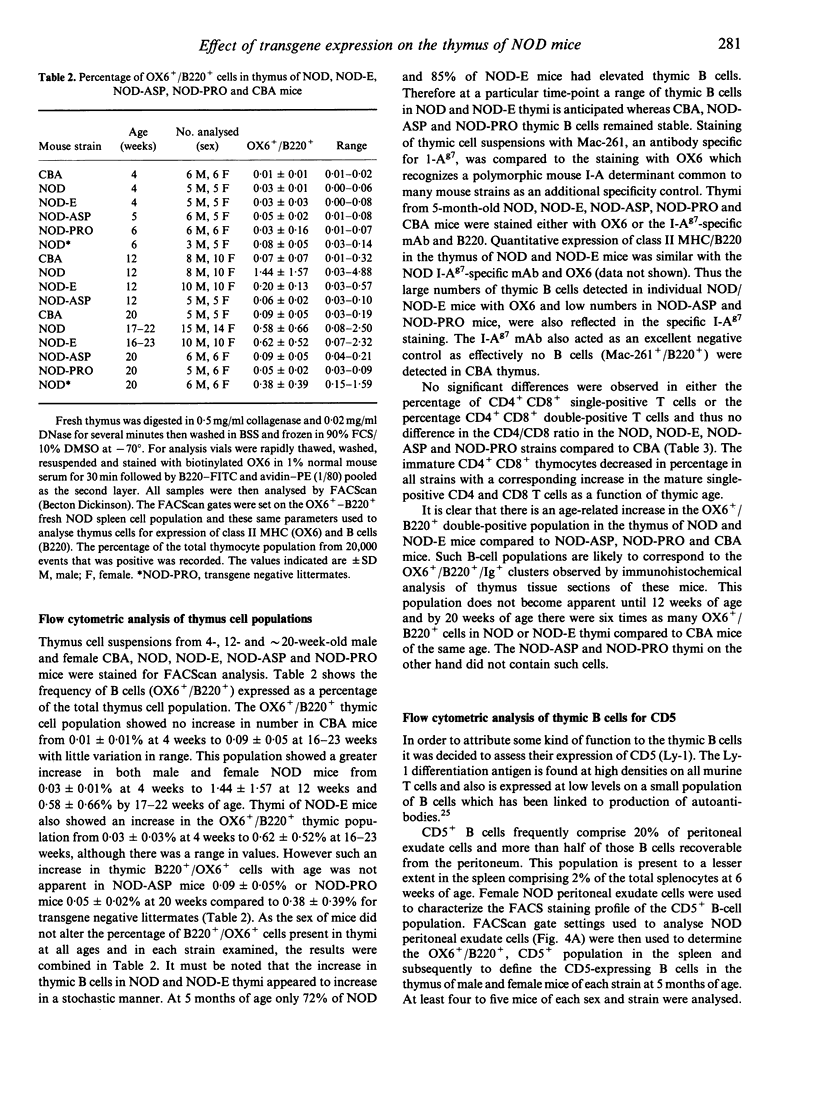
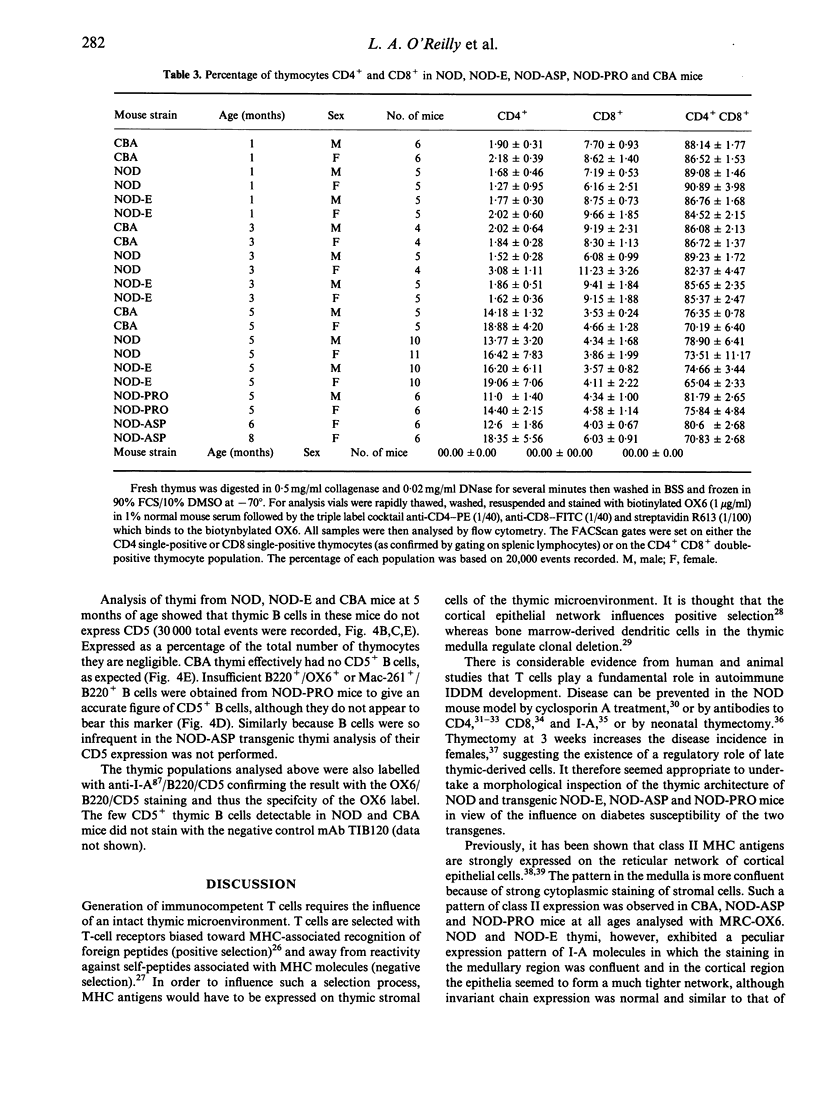
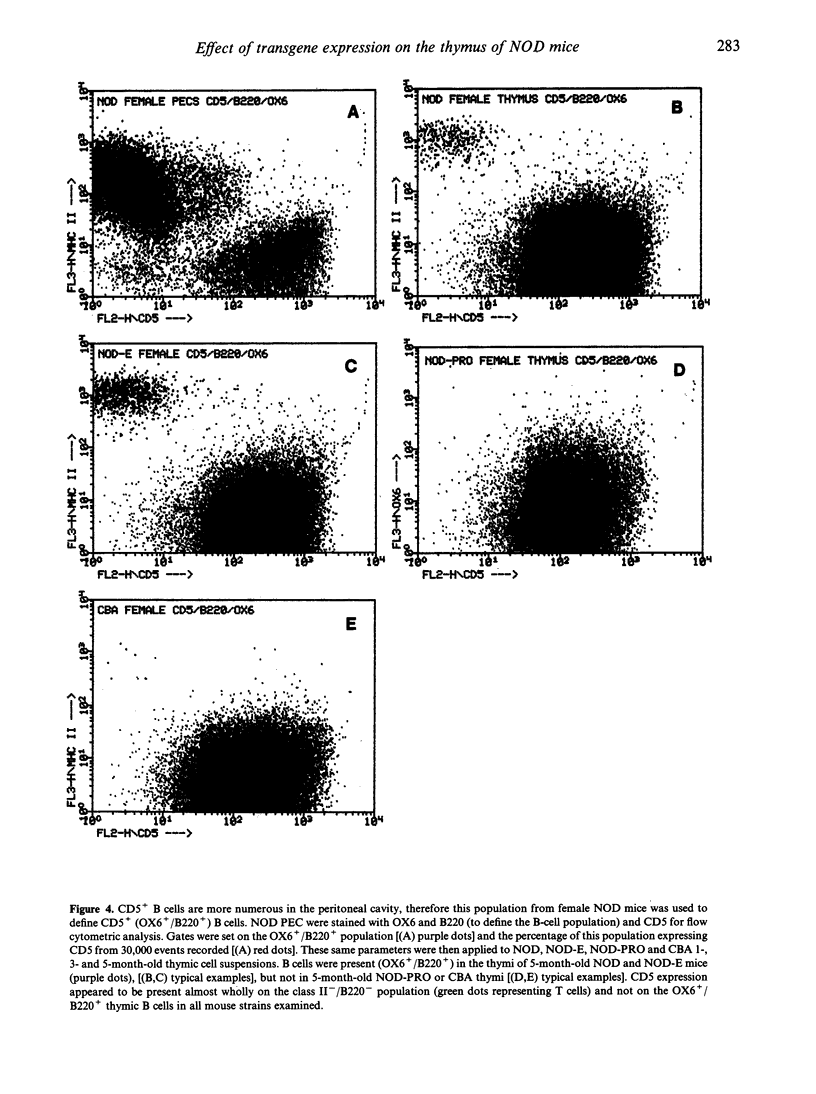
The non-obese diabetic (NOD) mouse is a good model of insulin-dependent diabetes mellitus. Autoreactive T cells may play a fundamental role in disease initiation in this model, while disregulation of such cells may result from an abnormal thymic microenvironment. Diabetes is prevented in NOD mice by direct introduction of an E alpha d transgene (NOD-E) or a modified I-A beta chain of NOD origin (NOD-PRO or NOD-ASP). To investigate if disease pathology in NOD mice, protection from disease in transgenic NOD-E and NOD-PRO and partial protection from disease in NOD-ASP can be attributed to alterations in the thymic microenvironment, immunohistochemical and flow cytometric analysis of the thymi of these mouse strains was studied. Thymi from NOD and NOD-E mice showed a progressive increase in thymic B-cell percentage from 12 weeks of age. This was accompanied by a concomitant loss in thymic epithelial cells with the appearance of large epithelial-free areas mainly at the corticomedullary junction, which increased in size and number with age and contained the B-cell clusters. Such thymic B cells did not express CD5 and were absent in CBA, NOD-ASP and NOD-PRO mice as were the epithelial cell-free spaces, even at 5 months of age. Therefore the mechanisms of disease protection in the transgenic NOD-E and NOD-ASP/NOD-PRO mice may differ if these thymic abnormalities are related to disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., McDevitt H. O. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu-Sánchez J. L., Faro J., Alonso J. M., Paige C. J., Martínez C., Marcos M. A. Ontogenic characterization of thymic B lymphocytes. Analysis in different mouse strains. Eur J Immunol. 1990 Aug;20(8):1767–1773. doi: 10.1002/eji.1830200822. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner R., Rijnbeek A. M., Bernabé R. R., Martinez-Alonso C., Coutinho A. Frequencies of background immunoglobulin-secreting cells in mice as a function if organ, age, and immune status. Immunobiology. 1981;158(3):225–238. doi: 10.1016/s0171-2985(81)80072-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Boitard C., Bendelac A., Richard M. F., Carnaud C., Bach J. F. Prevention of diabetes in nonobese diabetic mice by anti-I-A monoclonal antibodies: transfer of protection by splenic T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9719–9723. doi: 10.1073/pnas.85.24.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme J., Schuhbaur B., Kanagawa O., Benoist C., Mathis D. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990 Jul 20;249(4966):293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- Castaño L., Eisenbarth G. S. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Nakane P. K. Cells bearing Ia antigens in the murine thymus. An ultrastructural study. Am J Pathol. 1983 Apr;111(1):88–97. [PMC free article] [PubMed] [Google Scholar]
- Forsgren S., Dahl U., Söderström A., Holmberg D., Matsunaga T. The phenotype of lymphoid cells and thymic epithelium correlates with development of autoimmune insulitis in NOD in equilibrium with C57BL/6 allophenic chimeras. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9335–9339. doi: 10.1073/pnas.88.20.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N., Itoyama Y., Tabira T., Kuroiwa Y. Subsets of lymphoid cells in blood and thymus in myasthenia gravis. Monoclonal antibody analysis. J Neuroimmunol. 1983 Jun;4(3):151–159. doi: 10.1016/0165-5728(83)90031-0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986 Oct;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Hattori M., Buse J. B., Jackson R. A., Glimcher L., Dorf M. E., Minami M., Makino S., Moriwaki K., Kuzuya H., Imura H. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986 Feb 14;231(4739):733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- Holmes K. L., Morse H. C., 3rd Murine hematopoietic cell surface antigen expression. Immunol Today. 1988 Nov;9(11):344–350. doi: 10.1016/0167-5699(88)91335-7. [DOI] [PubMed] [Google Scholar]
- Hutchings P. R., Simpson E., O'Reilly L. A., Lund T., Waldmann H., Cooke A. The involvement of Ly2+ T cells in beta cell destruction. J Autoimmun. 1990 Apr;3 (Suppl 1):101–109. doi: 10.1016/s0896-8411(09)90018-x. [DOI] [PubMed] [Google Scholar]
- Hutchings P., O'Reilly L., Parish N. M., Waldmann H., Cooke A. The use of a non-depleting anti-CD4 monoclonal antibody to re-establish tolerance to beta cells in NOD mice. Eur J Immunol. 1992 Jul;22(7):1913–1918. doi: 10.1002/eji.1830220735. [DOI] [PubMed] [Google Scholar]
- Ikehara S., Tanaka H., Nakamura T., Furukawa F., Inoue S., Sekita K., Shimizu J., Hamashima Y., Good R. A. The influence of thymic abnormalities on the development of autoimmune diseases. Thymus. 1985;7(1):25–36. [PubMed] [Google Scholar]
- Inaba M., Inaba K., Hosono M., Kumamoto T., Ishida T., Muramatsu S., Masuda T., Ikehara S. Distinct mechanisms of neonatal tolerance induced by dendritic cells and thymic B cells. J Exp Med. 1991 Mar 1;173(3):549–559. doi: 10.1084/jem.173.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson E. J., Anderson G., Owen J. J. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med. 1992 Sep 1;176(3):845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanariou M., Huby R., Ladyman H., Colic M., Sivolapenko G., Lampert I., Ritter M. Immunosuppression with cyclosporin A alters the thymic microenvironment. Clin Exp Immunol. 1989 Nov;78(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Lo D., Sprent J. Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature. 1986 Feb 20;319(6055):672–675. doi: 10.1038/319672a0. [DOI] [PubMed] [Google Scholar]
- Lund T., O'Reilly L., Hutchings P., Kanagawa O., Simpson E., Gravely R., Chandler P., Dyson J., Picard J. K., Edwards A. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990 Jun 21;345(6277):727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- Lund T., Simpson E., Cooke A. Restriction fragment length polymorphisms in the major histocompatibility complex of the non-obese diabetic mouse. J Autoimmun. 1990 Jun;3(3):289–298. doi: 10.1016/0896-8411(90)90147-k. [DOI] [PubMed] [Google Scholar]
- MACKAY I. R., DEGAIL P. THYMIC "GERMINAL CENTRES" AND PLASMA CELLS IN SYSTEMIC LUPUS ERYTHEMATOSUS. Lancet. 1963 Sep 28;2(7309):667–667. doi: 10.1016/s0140-6736(63)90458-6. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K. Programmed death of autoreactive thymocytes. Nature. 1990 Feb 15;343(6259):642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- Manley S. W., Bourke J. R., Hawker R. W. The thyrotrophin receptor in guinea-pig thyroid homogenate: interaction with the long-acting thyroid stimulator. J Endocrinol. 1974 Jun;61(3):437–445. doi: 10.1677/joe.0.0610437. [DOI] [PubMed] [Google Scholar]
- Marrack P., McCormack J., Kappler J. Presentation of antigen, foreign major histocompatibility complex proteins and self by thymus cortical epithelium. Nature. 1989 Apr 6;338(6215):503–505. doi: 10.1038/338503a0. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Miyama-Inaba M., Kuma S., Inaba K., Ogata H., Iwai H., Yasumizu R., Muramatsu S., Steinman R. M., Ikehara S. Unusual phenotype of B cells in the thymus of normal mice. J Exp Med. 1988 Aug 1;168(2):811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina I. J., Cannon N. A., Hyman R., Huber B. T. Macrophages and T cells do not express Mlsa determinants. J Immunol. 1989 Jul 1;143(1):39–44. [PubMed] [Google Scholar]
- Mori Y., Suko M., Okudaira H., Matsuba I., Tsuruoka A., Sasaki A., Yokoyama H., Tanase T., Shida T., Nishimura M. Preventive effects of cyclosporin on diabetes in NOD mice. Diabetologia. 1986 Apr;29(4):244–247. doi: 10.1007/BF00454884. [DOI] [PubMed] [Google Scholar]
- Nabarra B., Andrianarison I. Thymic reticulum of autoimmune mice. I. Ultrastructural studies of the diabetic (db/db) mouse thymus. Exp Pathol. 1986;29(1):45–53. doi: 10.1016/s0232-1513(86)80005-6. [DOI] [PubMed] [Google Scholar]
- Nabarra B., Andrianarison I. Thymus reticulum of autoimmune mice. 3. Ultrastructural study of NOD (non-obese diabetic) mouse thymus. Int J Exp Pathol. 1991 Jun;72(3):275–287. [PMC free article] [PubMed] [Google Scholar]
- Nabarra B., Dardenne M., Bach J. F. Thymic reticulum of autoimmune mice. II: Ultrastructural studies of mice with lupus-like syndrome (NZB, BXSB, MRL/l). J Autoimmun. 1990 Feb;3(1):25–36. doi: 10.1016/0896-8411(90)90004-c. [DOI] [PubMed] [Google Scholar]
- Nishimoto H., Kikutani H., Yamamura K., Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. 1987 Jul 30-Aug 5Nature. 328(6129):432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- Parish N. M., Acha-Orbea H., Simpson E., Qin S. X., Lund T., Cooke A. A comparative study of T-cell receptor V beta usage in non-obese diabetic (NOD) and I-E transgenic NOD mice. Immunology. 1993 Apr;78(4):606–610. [PMC free article] [PubMed] [Google Scholar]
- Parish N. M., Chandler P., Quartey-Papafio R., Simpson E., Cooke A. The effect of bone marrow and thymus chimerism between non-obese diabetic (NOD) and NOD-E transgenic mice, on the expression and prevention of diabetes. Eur J Immunol. 1993 Oct;23(10):2667–2675. doi: 10.1002/eji.1830231042. [DOI] [PubMed] [Google Scholar]
- Patrick J., Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973 May 25;180(4088):871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Fowlkes B. J. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990 Jun 15;248(4961):1342–1348. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- Rouse R. V., van Ewijk W., Jones P. P., Weissman I. L. Expression of MHC antigens by mouse thymic dendritic cells. J Immunol. 1979 Jun;122(6):2508–2515. [PubMed] [Google Scholar]
- Savino W., Boitard C., Bach J. F., Dardenne M. Studies on the thymus in nonobese diabetic mouse. I. Changes in the microenvironmental compartments. Lab Invest. 1991 Mar;64(3):405–417. [PubMed] [Google Scholar]
- Savino W., Dardenne M. Developmental studies on expression of monoclonal antibody-defined cytokeratins by thymic epithelial cells from normal and autoimmune mice. J Histochem Cytochem. 1988 Sep;36(9):1123–1129. doi: 10.1177/36.9.2457046. [DOI] [PubMed] [Google Scholar]
- Scadding G. K., Vincent A., Newsom-Davis J., Henry K. Acetylcholine receptor antibody synthesis by thymic lymphocytes: correlation with thymic histology. Neurology. 1981 Aug;31(8):935–943. doi: 10.1212/wnl.31.8.935. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Banks-Schlegel S., Pinkus G. S. Immunohistochemical localization of keratin in normal human tissues. Lab Invest. 1980 Jan;42(1):91–96. [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Development of diabetogenic T cells from NOD/Lt marrow is blocked when an allo-H-2 haplotype is expressed on cells of hemopoietic origin, but not on thymic epithelium. J Immunol. 1991 Aug 15;147(4):1222–1229. [PubMed] [Google Scholar]
- Sharrow S. O., Mathieson B. J., Singer A. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J Immunol. 1981 Apr;126(4):1327–1335. [PubMed] [Google Scholar]
- Shizuru J. A., Taylor-Edwards C., Banks B. A., Gregory A. K., Fathman C. G. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988 Apr 29;240(4852):659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- Steinmann G. G., Klaus B., Müller-Hermelink H. K. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985 Nov;22(5):563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- T-cell repertoire. Immunol Rev. 1988 Jan;101:1–215. [PubMed] [Google Scholar]
- Uehira M., Uno M., Kürner T., Kikutani H., Mori K., Inomoto T., Uede T., Miyazaki J., Nishimoto H., Kishimoto T. Development of autoimmune insulitis is prevented in E alpha d but not in A beta k NOD transgenic mice. Int Immunol. 1989;1(2):209–213. doi: 10.1093/intimm/1.2.209. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Tanaka R., Nishimura T., Kumagai Y., Miyazaki J., Yamamura K., Habu S. I-E-restricted monoclonal expansion of B lymphocytes in the thymus of NOD mouse. Int Immunol. 1991 Aug;3(8):839–842. doi: 10.1093/intimm/3.8.839. [DOI] [PubMed] [Google Scholar]
- Weiss S., Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller M. Intrathymic presentation by dendritic cells and macrophages: their role in selecting T cells with specificity for internal and external nominal antigen. Immunology. 1991 Nov;74(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., MacKenzie W. A., Schuurman H. J., Ritter M. A., Price K. M., Broekhuizen R., Kater L. The human thymus microenvironment: heterogeneity detected by monoclonal anti-epithelial cell antibodies. Immunology. 1985 Apr;54(4):745–754. [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., Ron Y., Monaco J., Kappler J., Marrack P., Le Meur M., Gerlinger P., Durand B., Benoist C., Mathis D. Compartmentalization of MHC class II gene expression in transgenic mice. Cell. 1988 May 6;53(3):357–370. doi: 10.1016/0092-8674(88)90156-0. [DOI] [PubMed] [Google Scholar]