Abstract
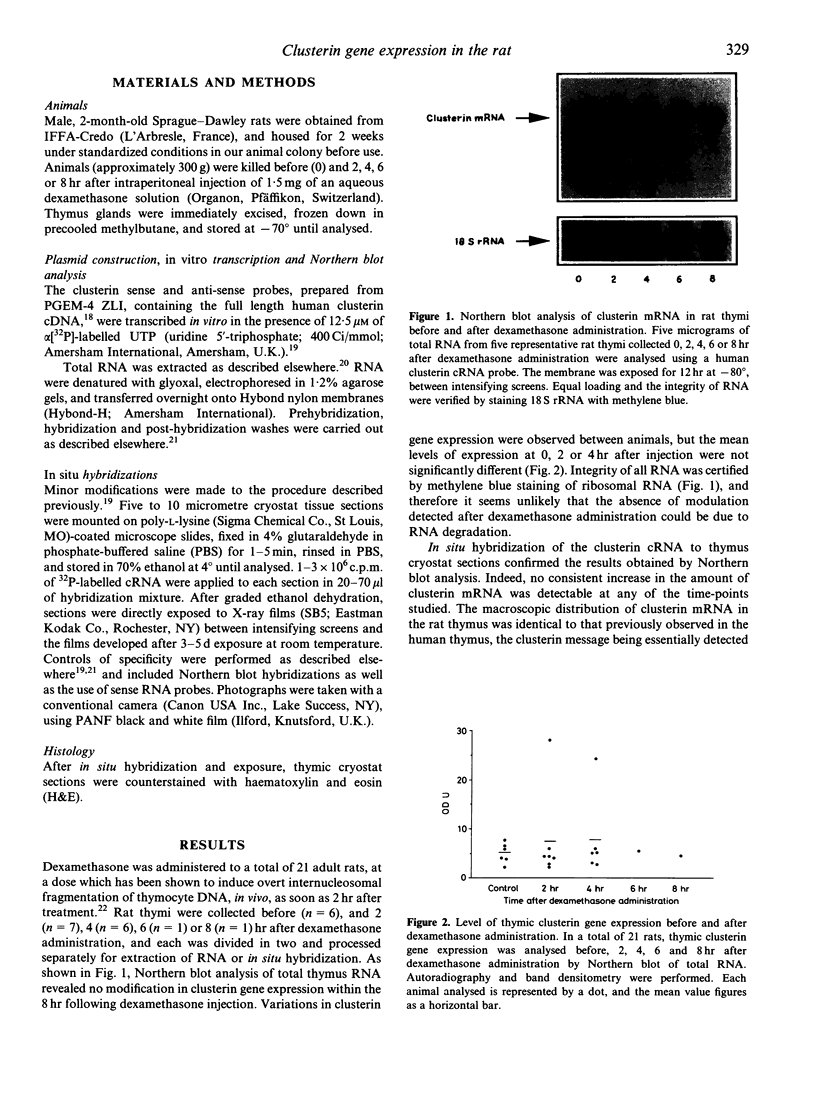
Clusterin, a multifunctional glycoprotein, characterized as a potent inhibitor of the membrane attack complex of complement, is also known to be the product of a gene that is highly up-regulated in certain tissues undergoing programmed cell death. We have studied the expression of this gene in the rat thymus after the induction of thymocyte programmed cell death (PCD) by in vivo dexamethasone administration. Northern blot analysis of clusterin mRNA 2, 4, 6 and 8 hr after dexamethasone administration in a total of 21 rats revealed no modification in the level of clusterin gene expression. In situ hybridization demonstrated that clusterin gene expression is macroscopically confined to the medullary region of the thymus, and that this distribution is not modified by dexamethasone administration. These results strongly suggest that in the rat, clusterin gene expression is not associated with the programmed cell death of thymocytes following in vivo dexamethasone administration. In situ hybridization of the clusterin cRNA to thymus cryostat sections confirmed the results obtained by Northern blot analysis. Indeed, no consistent increase in the amount of clusterin mRNA was detectable at any of the time-points studied. The macroscopic distribution of clusterin mRNA in the rat thymus was identical to that previously observed in the human thymus, clusterin message being essentially detected within the medullary regions. No modification in the macroscopic distribution of clusterin gene expression was detected after dexamethasone administration. These results suggest that, like the human thymus, medullary epithelial cells are the site of clusterin gene expression in the rat thymus. Moreover they indicate that an increase in the extent of ongoing thymocyte PCD does not significantly modify the rate or site of clusterin gene expression within the thymus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Litwack G. Glucocorticoid-induced lymphocytolysis is not mediated by an induced endonuclease. J Biol Chem. 1989 Mar 5;264(7):4104–4111. [PubMed] [Google Scholar]
- Bandyk M. G., Sawczuk I. S., Olsson C. A., Katz A. E., Buttyan R. Characterization of the products of a gene expressed during androgen-programmed cell death and their potential use as a marker of urogenital injury. J Urol. 1990 Feb;143(2):407–413. doi: 10.1016/s0022-5347(17)39975-5. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S., Troiano L., Davalli P., Tropea F., Ingletti M. C., Grassilli E., Monti D., Corti A., Franceschi C. In vivo accumulation of sulfated glycoprotein 2 mRNA in rat thymocytes upon dexamethasone-induced cell death. Biochem Biophys Res Commun. 1991 Mar 29;175(3):810–815. doi: 10.1016/0006-291x(91)91637-r. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Identification of a glucocorticoid-induced nuclease in thymocytes. A potential "lysis gene" product. J Biol Chem. 1987 Jun 15;262(17):8288–8292. [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Rapid in vivo effects of glucocorticoids on the integrity of rat lymphocyte genomic deoxyribonucleic acid. Endocrinology. 1986 Jan;118(1):38–45. doi: 10.1210/endo-118-1-38. [DOI] [PubMed] [Google Scholar]
- Connor J., Buttyan R., Olsson C. A., D'Agati V., O'Toole K., Sawczuk I. S. SGP-2 expression as a genetic marker of progressive cellular pathology in experimental hydronephrosis. Kidney Int. 1991 Jun;39(6):1098–1103. doi: 10.1038/ki.1991.139. [DOI] [PubMed] [Google Scholar]
- Dent A. L., Matis L. A., Hooshmand F., Widacki S. M., Bluestone J. A., Hedrick S. M. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990 Feb 22;343(6260):714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- French L. E., Sappino A. P., Tschopp J., Schifferli J. A. Distinct sites of production and deposition of the putative cell death marker clusterin in the human thymus. J Clin Invest. 1992 Nov;90(5):1919–1925. doi: 10.1172/JCI116069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French L. E., Wohlwend A., Sappino A. P., Tschopp J., Schifferli J. A. Human clusterin gene expression is confined to surviving cells during in vitro programmed cell death. J Clin Invest. 1994 Feb;93(2):877–884. doi: 10.1172/JCI117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima J., Zwain I., Lockshin R. A., Bardin C. W., Cheng C. Y. Diverse secretory patterns of clusterin by epididymis and prostate/seminal vesicles undergoing cell regression after orchiectomy. Endocrinology. 1990 Jun;126(6):2989–2997. doi: 10.1210/endo-126-6-2989. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Bosco D., Sappino A. P., Vassalli J. D. Plasminogen activator and mouse spermatozoa: urokinase synthesis in the male genital tract and binding of the enzyme to the sperm cell surface. J Cell Biol. 1987 May;104(5):1281–1289. doi: 10.1083/jcb.104.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992 Apr;17(4):154–159. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7123–7127. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Howe R. C., Pedrazzini T., Lees R. K., Budd R. C., Schneider R., Liao N. S., Zinkernagel R. M., Louis J. A., Raulet D. H. T-cell lineages, repertoire selection and tolerance induction. Immunol Rev. 1988 Aug;104:157–182. doi: 10.1111/j.1600-065x.1988.tb00762.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K. Programmed death of autoreactive thymocytes. Nature. 1990 Feb 15;343(6259):642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- May P. C., Lampert-Etchells M., Johnson S. A., Poirier J., Masters J. N., Finch C. E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990 Dec;5(6):831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Vassalli J. D., Belin D. Sites of synthesis of urokinase and tissue-type plasminogen activators in the murine kidney. J Clin Invest. 1991 Mar;87(3):962–970. doi: 10.1172/JCI115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensibar J. A., Griswold M. D., Sylvester S. R., Buttyan R., Bardin C. W., Cheng C. Y., Dudek S., Lee C. Prostatic ductal system in rats: regional variation in localization of an androgen-repressed gene product, sulfated glycoprotein-2. Endocrinology. 1991 Apr;128(4):2091–2102. doi: 10.1210/endo-128-4-2091. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G. Hormone-induced cell death. Purification ad properties of thymocytes undergoing apoptosis after glucocorticoid treatment. Am J Pathol. 1982 Oct;109(1):78–87. [PMC free article] [PubMed] [Google Scholar]
- de Silva H. V., Harmony J. A., Stuart W. D., Gil C. M., Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990 Jun 5;29(22):5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Teh H. S., Kisielow P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol Today. 1989 Feb;10(2):57–61. doi: 10.1016/0167-5699(89)90307-1. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]