Abstract
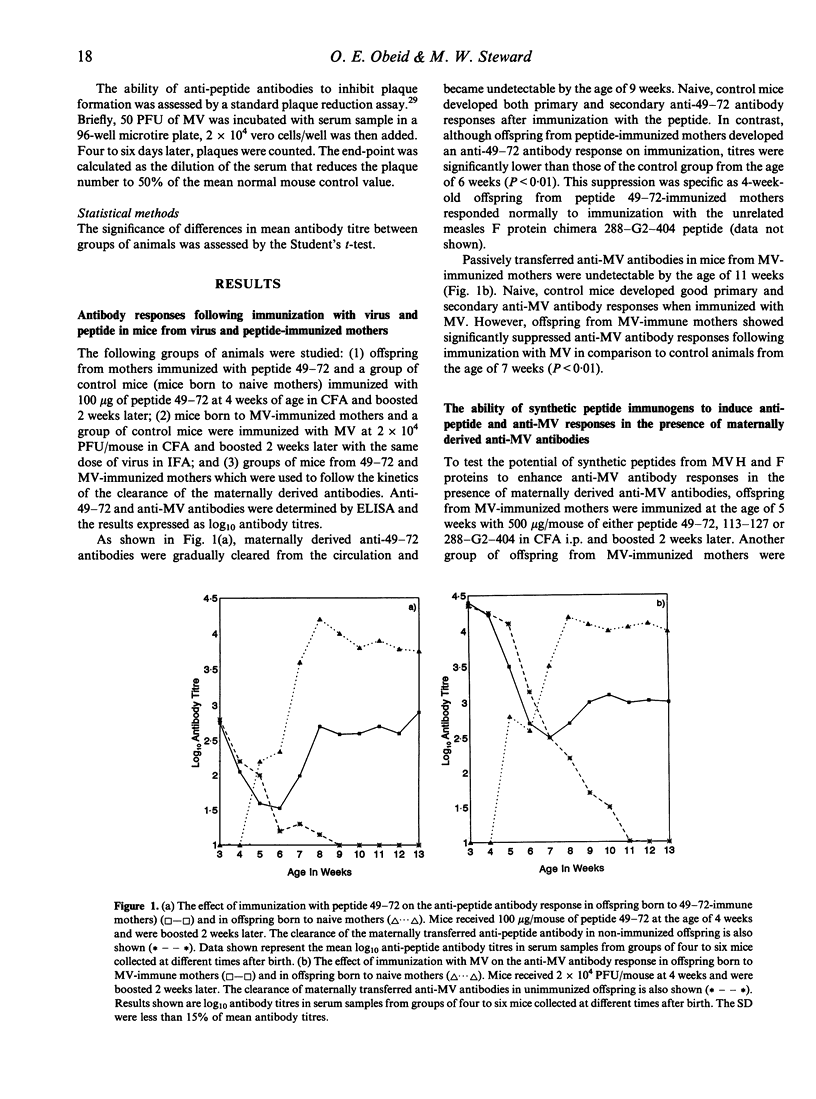
In this paper, we describe the results of experiments designed to test the hypothesis that immunogenic synthetic peptides representing non-immunodominant B- and T-cell epitopes of measles virus (MV) proteins can overcome the suppressive effect of maternal antibodies and induce anti-MV antibodies in young mice in the presence of maternal antibody to the virus. We have established a mouse model of immunosuppression by maternal antibody of both anti-MV and anti-peptide antibody responses in the young. Results obtained with this model immunization of young mice with a cocktail of synthetic peptides can overcome the suppression by maternal anti-MV antibodies and results in the induction of anti-peptide antibodies which recognize the virus. This work indicates that appropriately selected synthetic peptides have potential as vaccines which can be immunogenic and induce antibodies reactive with the virus-virus antibodies. in the presence of maternal anit-virus antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht P., Ennis F. A., Saltzman E. J., Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977 Nov;91(5):715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C. Neonatally induced tolerance to HGG: duration in B cells and absence of specific suppressor cells. J Immunol. 1977 Jul;119(1):311–314. [PubMed] [Google Scholar]
- Fernández I. M., Snijders A., Benaissa-Trouw B. J., Harmsen M., Snippe H., Kraaijeveld C. A. Influence of epitope polarity and adjuvants on the immunogenicity and efficacy of a synthetic peptide vaccine against Semliki Forest virus. J Virol. 1993 Oct;67(10):5843–5848. doi: 10.1128/jvi.67.10.5843-5848.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Yamaguchi N. Maternal T cells of immunized pregnant mice induce immune suppression in their offspring. Immunology. 1992 Oct;77(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Garenne M., Leroy O., Beau J. P., Sene I. Child mortality after high-titre measles vaccines: prospective study in Senegal. Lancet. 1991 Oct 12;338(8772):903–907. doi: 10.1016/0140-6736(91)91771-l. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Kunz H. W., Bernard C. F. Maternal-fetal interaction and immunological memory. Science. 1971 Jun 25;172(3990):1346–1348. doi: 10.1126/science.172.3990.1346. [DOI] [PubMed] [Google Scholar]
- Giraudon P., Wild T. F. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985 Jul 15;144(1):46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Ho P. C., Mutch D. A., Winkel K. D., Saul A. J., Jones G. L., Doran T. J., Rzepczyk C. M. Identification of two promiscuous T cell epitopes from tetanus toxin. Eur J Immunol. 1990 Mar;20(3):477–483. doi: 10.1002/eji.1830200304. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Hall E. IgE suppression by maternal IgG. Immunology. 1983 Jan;48(1):49–58. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Hall E. The development of IgE-suppressive immunocompetence in young animals: influence of exposure to antigen in the presence or absence of maternal immunity. Immunology. 1984 Oct;53(2):365–373. [PMC free article] [PubMed] [Google Scholar]
- Jelonek M. T., Brust J. L., Song C. H., Calandra G. B., Miller A., Sercarz E. E., Keller M. A. Maternal HEL immunization has no lasting effects on the immune response of offspring to immunization with hen egg-white lysozyme. Cell Immunol. 1993 May;148(2):422–434. doi: 10.1006/cimm.1993.1123. [DOI] [PubMed] [Google Scholar]
- Kindred B., Roelants G. E. Restricted clonal response to DNP in adult offspring of immunized mice: a maternal effect. J Immunol. 1974 Aug;113(2):445–448. [PubMed] [Google Scholar]
- Kuzu H., Kuzu Y., Zaghouani H., Bona C. In vivo priming effect during various stages of ontogeny of an influenza A virus nucleoprotein peptide. Eur J Immunol. 1993 Jun;23(6):1397–1400. doi: 10.1002/eji.1830230634. [DOI] [PubMed] [Google Scholar]
- McCullough K. C., Crowther J. R., Butcher R. N., Carpenter W. C., Brocchi E., Capucci L., De Simone F. Immune protection against foot-and-mouth disease virus studied using virus-neutralizing and non-neutralizing concentrations of monoclonal antibodies. Immunology. 1986 Jul;58(3):421–428. [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Tsutsumi H., Kumar N. S., Ogra P. L. Effect of breast feeding on the development of anti-idiotype antibody response to F glycoprotein of respiratory syncytial virus in infant mice after post-partum maternal immunization. J Immunol. 1989 Apr 1;142(7):2507–2512. [PubMed] [Google Scholar]
- Parekh B. S., Shaffer N., Pau C. P., Abrams E., Thomas P., Pollack H., Bamji M., Kaul A., Schochetman G., Rogers M. Lack of correlation between maternal antibodies to V3 loop peptides of gp120 and perinatal HIV-1 transmission. The NYC Perinatal HIV Transmission Collaborative Study. AIDS. 1991 Oct;5(10):1179–1184. doi: 10.1097/00002030-199110000-00004. [DOI] [PubMed] [Google Scholar]
- Partidos C. D., Obeid O. E., Steward M. W. Antibody responses to non-immunogenic synthetic peptides induced by co-immunization with immunogenic peptides. Immunology. 1992 Oct;77(2):262–266. [PMC free article] [PubMed] [Google Scholar]
- Partidos C., Stanley C., Steward M. The influence of orientation and number of copies of T and B cell epitopes on the specificity and affinity of antibodies induced by chimeric peptides. Eur J Immunol. 1992 Oct;22(10):2675–2680. doi: 10.1002/eji.1830221030. [DOI] [PubMed] [Google Scholar]
- Peppard J. V. Feeding neonatal rats with IgG antibodies leads to humoral hyporesponsiveness in the adult. Immunology. 1992 Oct;77(2):256–261. [PMC free article] [PubMed] [Google Scholar]
- Peppard J. V., Jackson L. E., Hall J. G., Robertson D. The transfer of immune complexes from the lumen of the small intestine to the bloodstream in sucking rats. Immunology. 1984 Oct;53(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Peppard J. V., Jackson L. E., Mackenzie N. M. Comparison of in vitro binding to enterocyte brush borders of rat IgG subclasses and their transmission in vivo in the rat. Immunology. 1985 Sep;56(1):51–55. [PMC free article] [PubMed] [Google Scholar]
- Peri B. A., Rothberg R. M. Specific suppression of antibody production in young rabbit kits after maternal ingestion of bovine serum albumin. J Immunol. 1981 Dec;127(6):2520–2525. [PubMed] [Google Scholar]
- Roberts S. A., Turner M. W. Specific suppression of rat IgE responses with milk from immunized females and with feeds of serum antibody. Immunology. 1983 Jan;48(1):195–199. [PMC free article] [PubMed] [Google Scholar]
- Rothstein T. L., Vastola A. P. Homologous monoclonal antibodies induce idiotope-specific suppression in neonates through maternal influence and in adults exposed during fetal and neonatal life. J Immunol. 1984 Sep;133(3):1151–1154. [PubMed] [Google Scholar]
- Sarvas H., Kurikka S., Seppälä I. J., Mäkelä P. H., Mäkelä O. Maternal antibodies partly inhibit an active antibody response to routine tetanus toxoid immunization in infants. J Infect Dis. 1992 May;165(5):977–979. doi: 10.1093/infdis/165.5.977. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Ono Y., Ishida N. Modification of fetal immune system by maternal anti-DNA antibody. I. Enhanced immune response to DNA in the mice exposed to anti-DNA antibody early in life. J Immunol. 1977 Jul;119(1):26–30. [PubMed] [Google Scholar]
- Shaw D. M., Stanley C. M., Partidos C. D., Steward M. W. Influence of the T-helper epitope on the titre and affinity of antibodies to B-cell epitopes after co-immunization. Mol Immunol. 1993 Aug;30(11):961–968. doi: 10.1016/0161-5890(93)90121-q. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Song C. H., Calandra G. B., Palmer C. J., Miller A., Sercarz E. E., Keller M. A. Inhibition of offspring response to HEL-CFA by administration of anti-HEL MAB to the mother is not related to the predominant idiotype, IdXE, or specificity of the MAB. Cell Immunol. 1990 Dec;131(2):311–324. doi: 10.1016/0008-8749(90)90257-r. [DOI] [PubMed] [Google Scholar]
- Trudel M., Nadon F., Séguin C., Binz H. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology. 1991 Dec;185(2):749–757. doi: 10.1016/0042-6822(91)90546-n. [DOI] [PubMed] [Google Scholar]
- Uher F., Dickler H. B. Cooperativity between B lymphocyte membrane molecules: independent ligand occupancy and cross-linking of antigen receptors and Fc gamma receptors down-regulates B lymphocyte function. J Immunol. 1986 Nov 15;137(10):3124–3129. [PubMed] [Google Scholar]
- Uher F., Lamers M. C., Dickler H. B. Antigen-antibody complexes bound to B-lymphocyte Fc gamma receptors regulate B-lymphocyte differentiation. Cell Immunol. 1985 Oct 15;95(2):368–379. doi: 10.1016/0008-8749(85)90324-7. [DOI] [PubMed] [Google Scholar]
- Yeager A. S., Davis J. H., Ross L. A., Harvey B. Measles immunization. Successes and failures. JAMA. 1977 Jan 24;237(4):347–351. [PubMed] [Google Scholar]


