Abstract
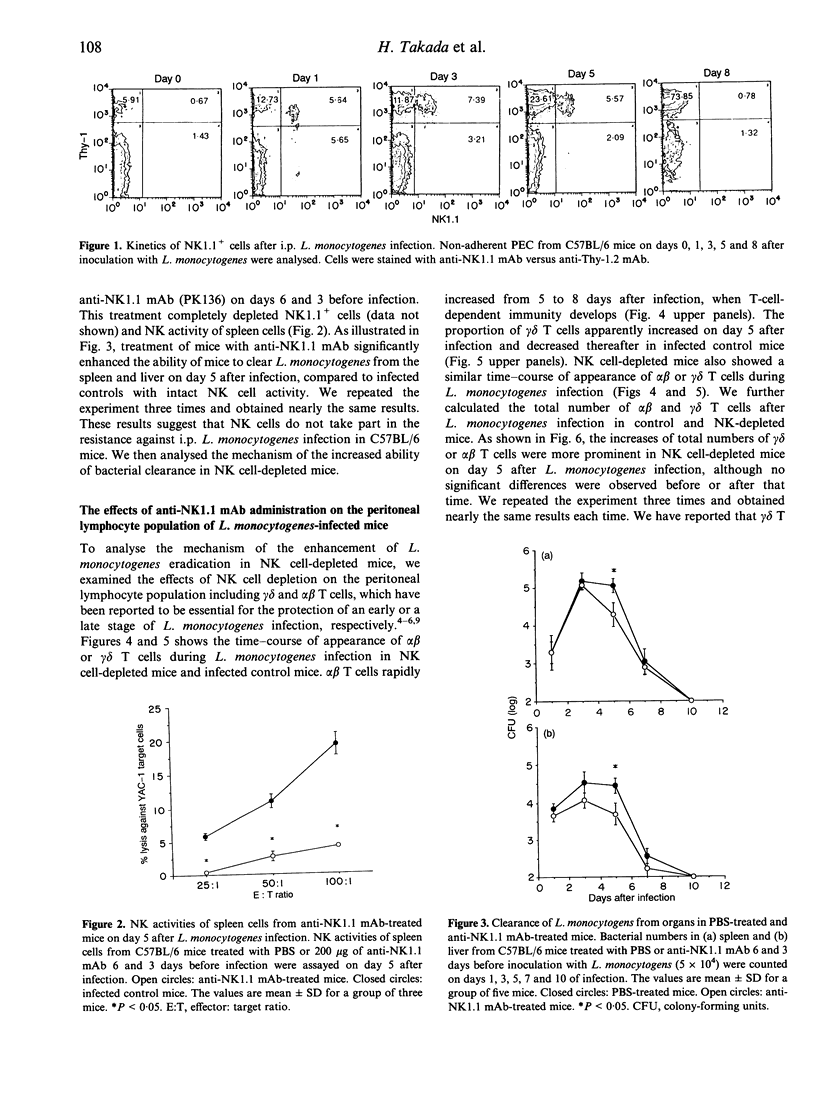
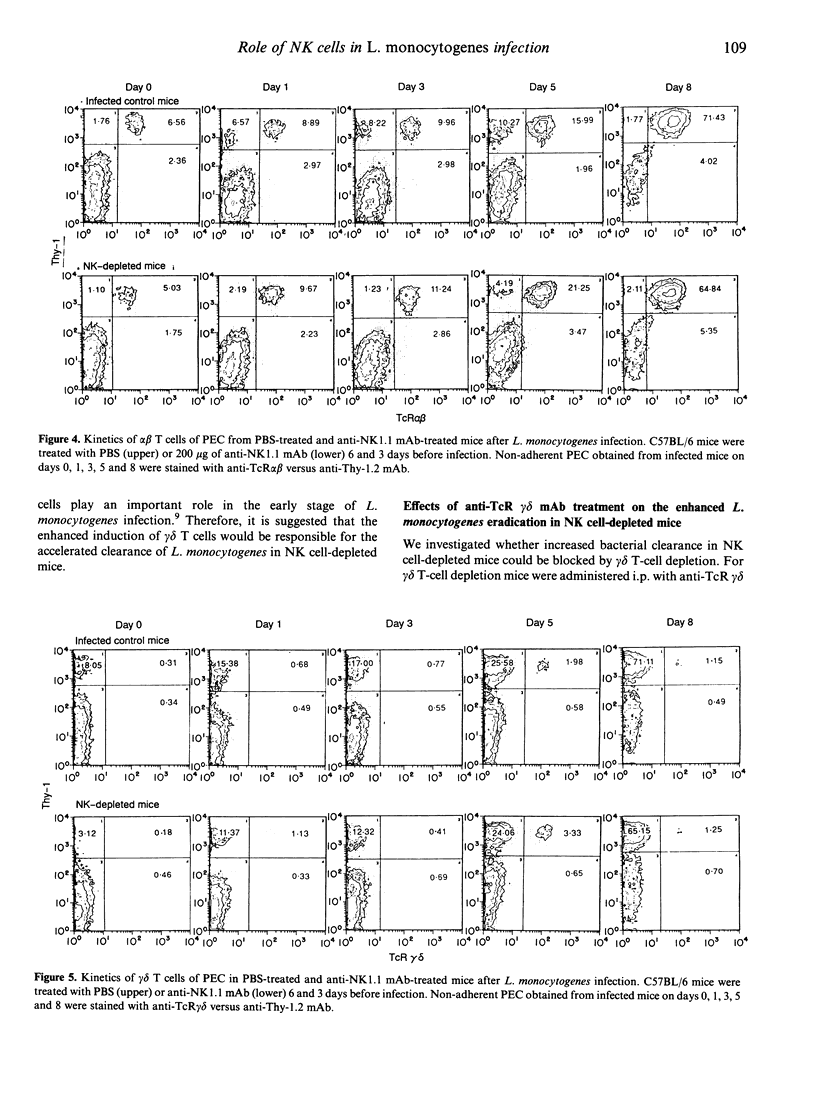
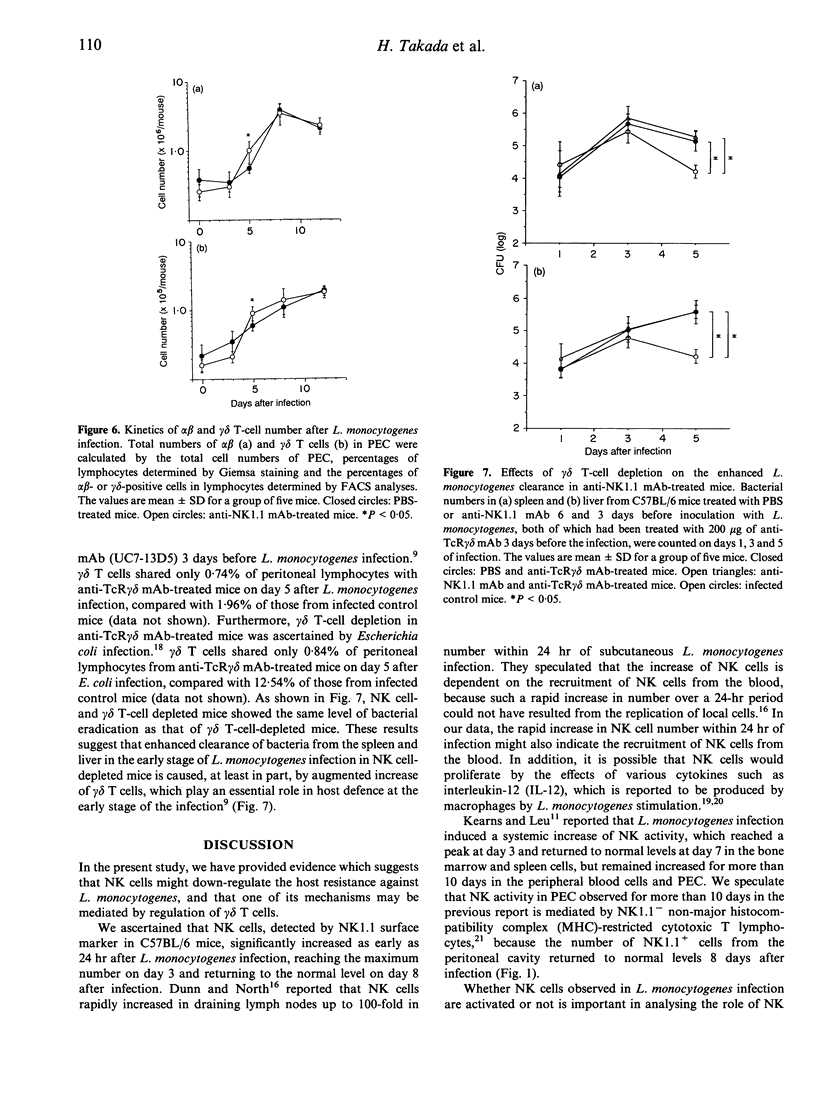
We have reported that T cells bearing T-cell receptors (TcR) of gamma delta type (gamma delta T cells) appear in the peritoneal cavity in a relatively early stage of primary intraperitoneal (i.p.) Listeria monocytogenes infection, and play a significant role against the infection. To elucidate the protective role of natural killer cells which also appear in the early stage of L. monocytogenes infection, mice were treated with anti-NK1.1 monoclonal antibody (mAb) to deplete NK cells before the infection. They exhibited accelerated clearance of L. monocytogenes, accompanied by enhanced induction of gamma delta T cells in the peritoneal cavity compared with non-treated mice. When the mice were depleted of gamma delta T cells by in vivo administration of anti-TcR gamma delta mAb, the bacterial burdens of organs from infected mice were not affected by NK cell depletion. These results suggest that, although NK cells increase significantly during the early stage of L. monocytogenes infection, they do not take part in the early host resistance against i.p. L. monocytogenes infection. It is also suggested that increased gamma delta T cells in the peritoneal cavity of NK cell-depleted mice can be one of the factors responsible for the enhanced clearance of L. monocytogenes in the early stage of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Habu S., Okumura K., Welsh R. M. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983 Sep;131(3):1531–1538. [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Welsh R. M. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984 Oct;52(1):119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Wood P. Listeriosis in beige mice and their heterozygous littermates. Immunology. 1984 Apr;51(4):711–717. [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Peñarrubia P., Koster F. T., Kelley R. O., McDowell T. D., Bankhurst A. D. Antibacterial activity of human natural killer cells. J Exp Med. 1989 Jan 1;169(1):99–113. doi: 10.1084/jem.169.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S., Akamatsu K., Tamaoki N., Okumura K. In vivo significance of NK cell on resistance against virus (HSV-1) infections in mice. J Immunol. 1984 Nov;133(5):2743–2747. [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Destruction of Trypanosoma cruzi by Natural killer cells. Science. 1982 Oct 15;218(4569):295–296. doi: 10.1126/science.6812218. [DOI] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Tsai V. Acute toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J Immunol. 1986 Jan;136(1):313–319. [PubMed] [Google Scholar]
- Holmberg L. A., Springer T. A., Ault K. A. Natural killer activity in the peritoneal exudates of mice infected with Listeria monocytogenes: characterization of the natural killer cells by using a monoclonal rat anti-murine macrophage antibody (M1/70). J Immunol. 1981 Nov;127(5):1792–1799. [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Jimenez B. E., Murphy J. W. In vitro effects of natural killer cells against Paracoccidioides brasiliensis yeast phase. Infect Immun. 1984 Nov;46(2):552–558. doi: 10.1128/iai.46.2.552-558.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity against intracellular bacteria: biological effector functions and antigen specificity of T lymphocytes. Curr Top Microbiol Immunol. 1988;138:141–176. [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Regulatory interactions between macrophages and T-cell subsets in Listeria monocytogenes-specific T-cell activation. Infect Immun. 1982 Dec;38(3):907–913. doi: 10.1128/iai.38.3.907-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns R. J., Leu R. W. Modulation of natural killer activity in mice following infection with Listeria monocytogenes. Cell Immunol. 1984 Apr 1;84(2):361–371. doi: 10.1016/0008-8749(84)90108-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongshavn P. A., Skamene E. The role of natural resistance in protection of the murine host from listeriosis. Clin Invest Med. 1984;7(4):253–257. [PubMed] [Google Scholar]
- Kratz S. S., Kurlander R. J. Characterization of the pattern of inflammatory cell influx and cytokine production during the murine host response to Listeria monocytogenes. J Immunol. 1988 Jul 15;141(2):598–606. [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H., Hackett J., Jr, Tutt M., Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986 Nov 1;137(9):2735–2739. [PubMed] [Google Scholar]
- Lukacs K., Kurlander R. Lyt-2+ T cell-mediated protection against listeriosis. Protection correlates with phagocyte depletion but not with IFN-gamma production. J Immunol. 1989 Apr 15;142(8):2879–2886. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Dempsey W. L., Volkman A., Connor J. Antimicrobial activity of various immunomodulators: independence from normal levels of circulating monocytes and natural killer cells. Infect Immun. 1986 Jan;51(1):87–93. doi: 10.1128/iai.51.1.87-93.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983 Feb;130(2):903–907. [PubMed] [Google Scholar]
- Ohga S., Yoshikai Y., Takeda Y., Hiromatsu K., Nomoto K. Sequential appearance of gamma/delta- and alpha/beta-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur J Immunol. 1990 Mar;20(3):533–538. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- Schultheis R. J., Kearns R. J. In vivo administration of anti-asialo-GM1 antibody enhances splenic clearance of Listeria monocytogenes. Nat Immun Cell Growth Regul. 1990;9(6):376–386. [PubMed] [Google Scholar]
- Shah P. D. Dendritic cells but not macrophages are targets for immune regulation by natural killer cells. Cell Immunol. 1987 Feb;104(2):440–445. doi: 10.1016/0008-8749(87)90046-3. [DOI] [PubMed] [Google Scholar]
- Shah P. D., Gilbertson S. M., Rowley D. A. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985 Aug 1;162(2):625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Hiromatsu K., Matsuzaki G., Muramori K., Nomoto K. Peritoneal gamma delta T cells induced by Escherichia coli infection in mice. Correlation between Thy-1 phenotype and host minor lymphocyte-stimulating phenotype. J Immunol. 1993 Aug 15;151(4):2062–2069. [PubMed] [Google Scholar]
- Tutt M. M., Kuziel W. A., Hackett J., Jr, Bennett M., Tucker P. W., Kumar V. Murine natural killer cells do not express functional transcripts of the alpha-, beta-, or gamma-chain genes of the T cell receptor. J Immunol. 1986 Nov 1;137(9):2998–3001. [PubMed] [Google Scholar]


