Abstract
Autoimmune prostatitis developed spontaneously in (C57BL/6N x A/J)F1 (B6A) mice, when thymectomy (Tx) was conducted on day 3 after birth (Tx-3). The lesion could be prevented by a single injection of CD4+ spleen cells (4 x 10(6)) from normal males, but not from normal females or newborn orchidectomized (Orx-0) mice. However, when spleen cells were obtained from Orx-0 mice that had received a dihydrotestosterone (DHT) pellet when adult to develop a mature prostate, prostatitis could be prevented, suggesting that immune tolerance to prostate antigen(s) is maintained by a CD4+ tissue-specific suppressor T cell (Ts) population(s), which is activated by a specific autoantigen(s) in the mature prostate. The result that even CD4+ cells from Orx-0 mice that were thymectomized as adults and treated thereafter with DHT were effective for prevention of prostatitis suggests that activation of this Ts population takes place in the peripheral lymphoid organs, and that it maintains peripheral tolerance to autoantigen in the prostate of these mice and probably also in normal mice.
Full text
PDF

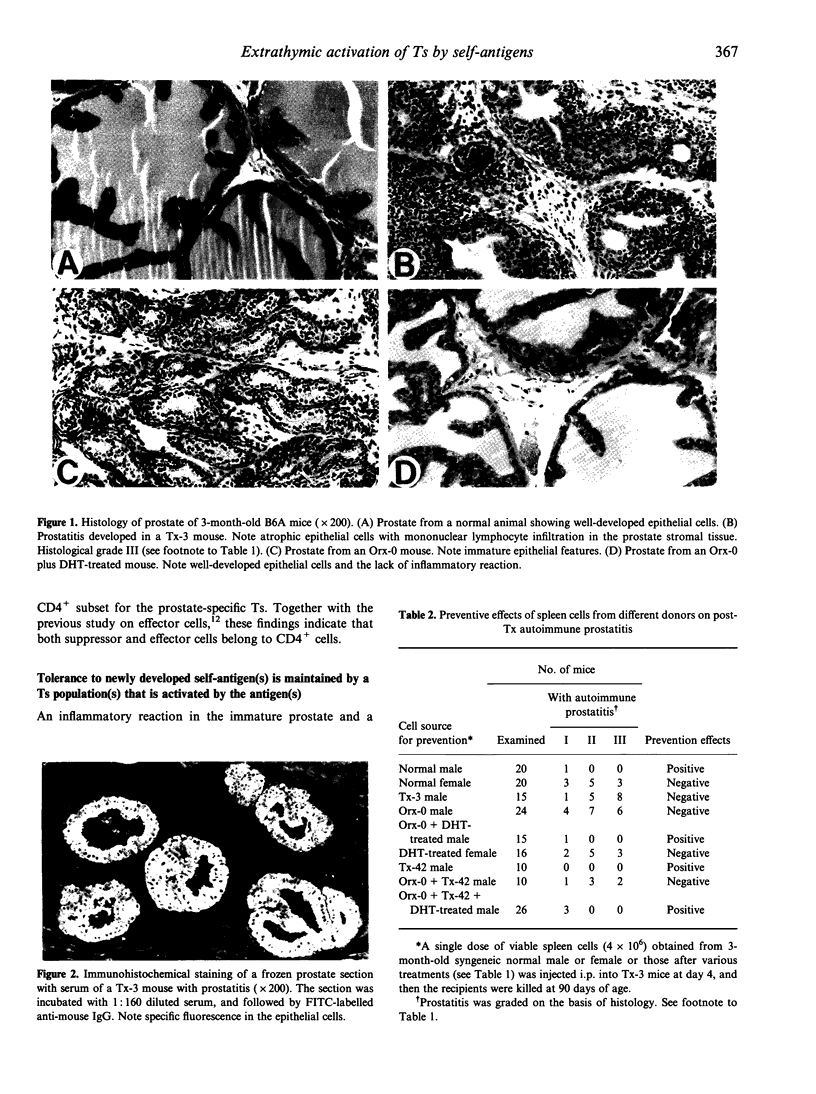


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Campbell I. L., Morahan G., Mandel T. E., Harrison L. C., Miller J. F. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988 Jun 9;333(6173):529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Hong R., Schulte-Wissermann H., Jarrett-Toth E., Horowitz S. D., Manning D. D. Transplantation of cultured thymic fragments. II. Results in nude mice. J Exp Med. 1979 Feb 1;149(2):398–415. doi: 10.1084/jem.149.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Taguchi O., Takahashi T., Itoh G., Nishizuka Y. L3T4 effector cells in multiple organ-localized autoimmune disease in nude mice grafted with embryonic rat thymus. J Exp Med. 1988 Dec 1;168(6):2397–2402. doi: 10.1084/jem.168.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaak D. D. Fate of skin grafts from different inbred strains on nude mice bearing allogeneic thymus grafts. J Reticuloendothel Soc. 1978 Mar;23(3):231–233. [PubMed] [Google Scholar]
- Itoh M., Mukasa A., Tokunaga Y., Hiramine C., Hojo K. Suppression of efferent limb of testicular autoimmune response by a regulatory CD4+ T cell line in mice. Clin Exp Immunol. 1992 Mar;87(3):455–460. doi: 10.1111/j.1365-2249.1992.tb03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Merriam G. R., Nelson L. M., Kruisbeck A. M. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J Exp Med. 1990 Nov 1;172(5):1277–1285. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kojima A., Prehn R. T. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14(1-2):15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Widera G., Cowing C., Flavell R. A., Palmiter R. D., Brinster R. L. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988 Apr 8;53(1):159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Murakami K., Maruyama H., Nishio A., Kuribayashi K., Inaba M., Inaba K., Hosono M., Shinagawa K., Sakai M., Masuda T. Effects of intrathymic injection of organ-specific autoantigens, parietal cells, at the neonatal stage on autoreactive effector and suppressor T cell precursors. Eur J Immunol. 1993 Apr;23(4):809–814. doi: 10.1002/eji.1830230406. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Fowlkes B. J. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990 Jun 15;248(4961):1342–1348. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- Salaün J., Bandeira A., Khazaal I., Calman F., Coltey M., Coutinho A., Le Douarin N. M. Thymic epithelium tolerizes for histocompatibility antigens. Science. 1990 Mar 23;247(4949 Pt 1):1471–1474. doi: 10.1126/science.247.4949.1471. [DOI] [PubMed] [Google Scholar]
- Smith H., Sakamoto Y., Kasai K., Tung K. S. Effector and regulatory cells in autoimmune oophoritis elicited by neonatal thymectomy. J Immunol. 1991 Nov 1;147(9):2928–2933. [PubMed] [Google Scholar]
- Taguchi O., Kojima A., Nishizuka Y. Experimental autoimmune prostatitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1985 Apr;60(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J Exp Med. 1987 Jan 1;165(1):146–156. doi: 10.1084/jem.165.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Takahashi T., Nishizuka Y. Self-tolerance and localized autoimmunity. Curr Opin Immunol. 1989;2(4):576–581. doi: 10.1016/0952-7915(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Taguchi O., Takahashi T., Seto M., Namikawa R., Matsuyama M., Nishizuka Y. Development of multiple organ-localized autoimmune diseases in nude mice after reconstitution of T cell function by rat fetal thymus graft. J Exp Med. 1986 Jul 1;164(1):60–71. doi: 10.1084/jem.164.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]




