Abstract
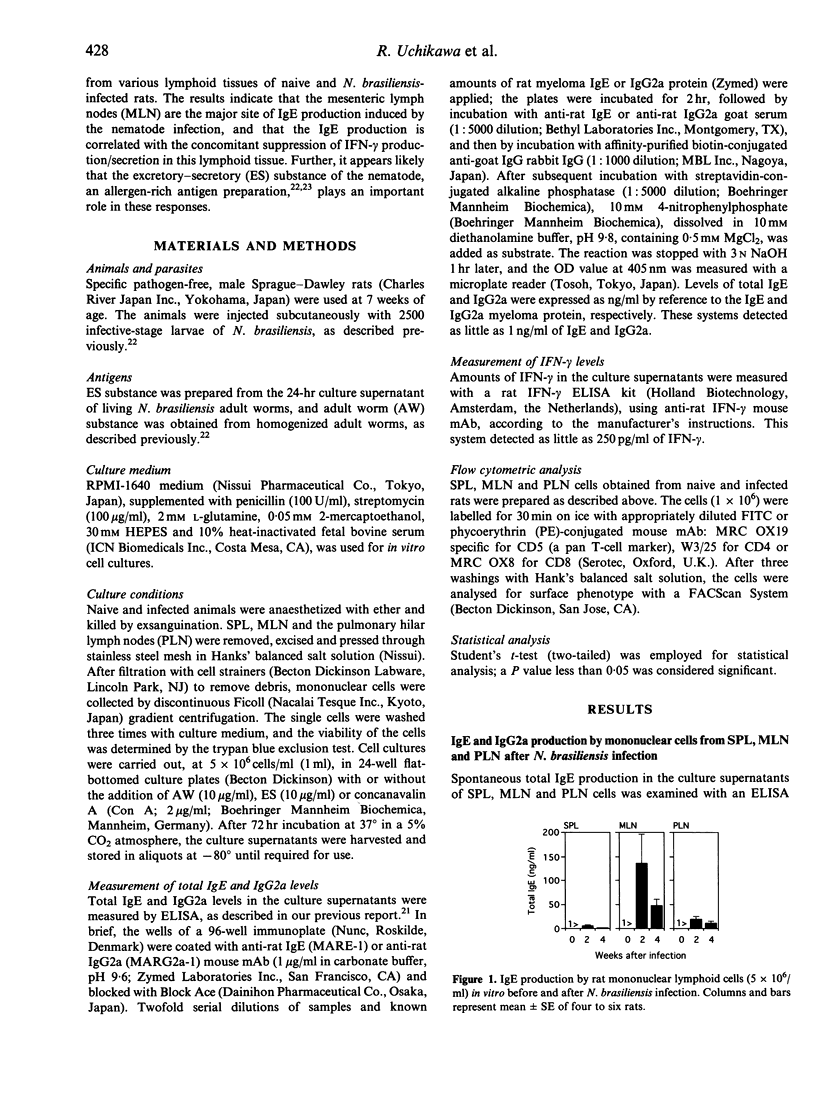
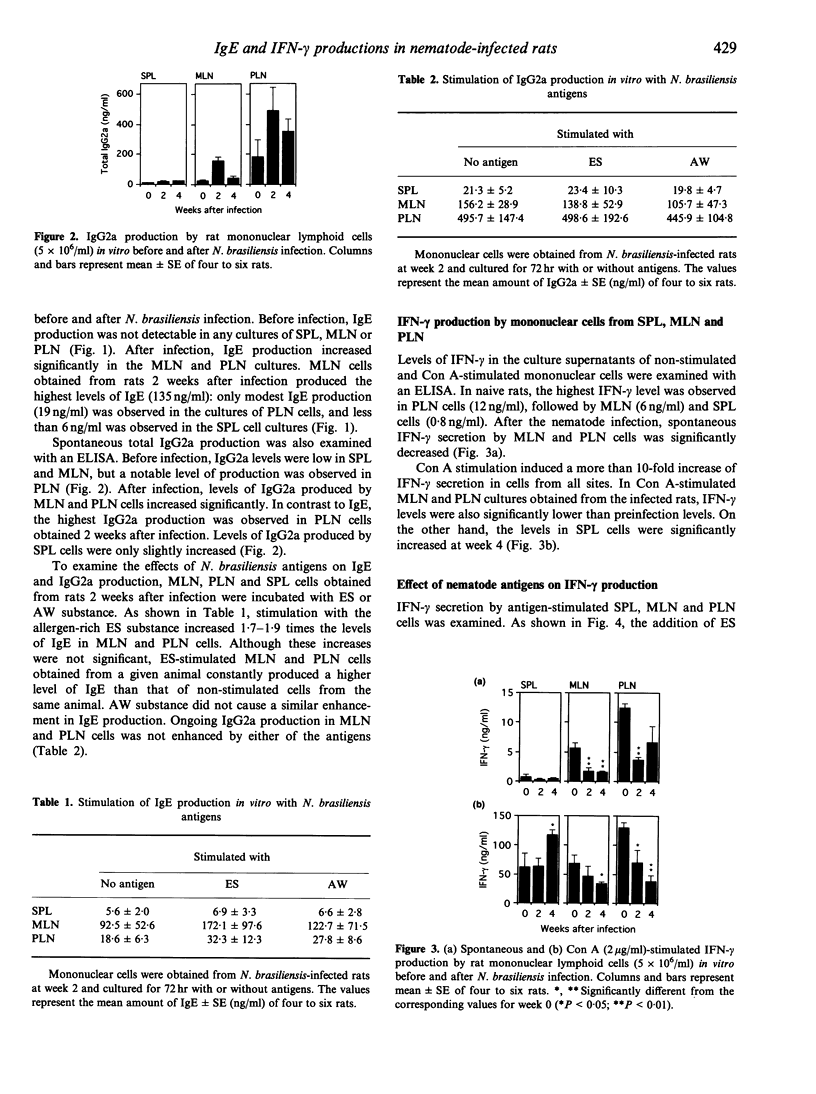
IgE and IgG2a antibody production and interferon (IFN)-gamma secretion were studied in rats infected with the gut nematode Nippostrongylus brasiliensis by in vitro cultivation of mononuclear cells obtained from spleen (SPL), mesenteric lymph nodes (MLN) and pulmonary hilar lymph nodes (PLN). The highest levels of IgE were detected in the culture supernatants of MLN cells after infection: IgE levels were modest in PLN and negligible in SPL. In contrast, the highest levels of IgG2a were produced by PLN cells, followed by MLN and SPL cells. These results indicate that the MLN is the most significant site for IgE production in nematode infection, while IgG2a production is more marked in PLN. In naive rats, the spontaneous secretion of IFN-gamma was highest in PLN cells, followed by MLN and SPL cells. After the infection, IFN-gamma levels were significantly decreased in MLN and PLN. Suppression of IFN-gamma secretion was also observed in concanavalin A (ConA)-stimulated MLN and PLN cells from infected rats. In MLN, the ratio of CD4+ to CD8+ T cells was increased after the infection. Stimulation with an allergen-rich, excretory-secretory (ES) substance of the nematode enhanced ongoing IgE production, and suppressed IFN-gamma secretion by MLN and PLN cells. In contrast, an allergen-poor, adult worm extract potentiated IFN-gamma secretion. These results show that nematode-induced IgE antibody response is associated with the suppressed production and/or secretion of IFN-gamma, particularly in the MLN, and that some molecules in the ES substance may trigger these immune responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan W., Mayrhofer G. Origins of serum IgE and of the homocytotropic antibody-secreting cells in the thoracic duct lymph of rats infested with Nippostrongylus brasiliensis. Int Arch Allergy Appl Immunol. 1984;74(3):270–273. doi: 10.1159/000233556. [DOI] [PubMed] [Google Scholar]
- Auci D. L., Chice S. M., Heusser C., Athanassiades T. J., Durkin H. G. Origin and fate of IgE-bearing lymphocytes. II. Gut-associated lymphoid tissue as sites of first appearance of IgE-bearing B lymphocytes and hapten-specific IgE antibody-forming cells in mice immunized with benzylpenicilloyl-keyhole limpet hemocyanin by various routes: relation to asialo GM1 ganglioside+ cells and IgE/CD23 immune complexes. J Immunol. 1992 Oct 1;149(7):2241–2248. [PubMed] [Google Scholar]
- Bakhiet M., Olsson T., van der Meide P., Kristensson K. Depletion of CD8+ T cells suppresses growth of Trypanosoma brucei brucei and interferon-gamma) production in infected rats. Clin Exp Immunol. 1990 Aug;81(2):195–199. doi: 10.1111/j.1365-2249.1990.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn C. C., Selkirk M. E. Inactivation of platelet-activating factor by a putative acetylhydrolase from the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunology. 1992 Jan;75(1):41–46. [PMC free article] [PubMed] [Google Scholar]
- Burt J. S., Ogilvie B. M. In vitro maintenance of nematode parasites assayed by acetylcholinesterase and allergen secretion. Exp Parasitol. 1975 Aug;38(1):75–82. doi: 10.1016/0014-4894(75)90039-9. [DOI] [PubMed] [Google Scholar]
- Cardell S., Sander B., Möller G. Primary stimulation of CD4+ cells in the presence of IL-4 or IFN-gamma alters the frequencies of cytokine-producing cells at restimulation. Scand J Immunol. 1992 Dec;36(6):769–777. doi: 10.1111/j.1365-3083.1992.tb03138.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. The sensitivity of rat CD8+ and CD4+ T cells to ricin in vivo and in vitro and their relationship to IgE regulation. Immunology. 1990 Jan;69(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Lee T. H., Kemeny D. M. Ricin enhances IgE responses by inhibiting a subpopulation of early-activated IgE regulatory CD8+ T cells. Immunology. 1993 Feb;78(2):226–236. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Noble A., Staynov D. Z., Lee T. H., Kemeny D. M. Elimination of IgE regulatory rat CD8+ T cells in vivo differentially modulates interleukin-4 and interferon-gamma but not interleukin-2 production by splenic T cells. Immunology. 1993 Apr;78(4):513–519. [PMC free article] [PubMed] [Google Scholar]
- Ding L., Shevach E. M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992 May 15;148(10):3133–3139. [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Urban J. F., Jr Cytokines: making the right choice. Parasitol Today. 1992 Sep;8(9):311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- Jarrett E. E., Haig D. M. Time course studies on rat IgE production in N. Brasiliensis infection. Clin Exp Immunol. 1976 May;24(2):346–351. [PMC free article] [PubMed] [Google Scholar]
- Jujo K., Renz H., Abe J., Gelfand E. W., Leung D. Y. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 1):323–331. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- King C. L., Low C. C., Nutman T. B. IgE production in human helminth infection. Reciprocal interrelationship between IL-4 and IFN-gamma. J Immunol. 1993 Mar 1;150(5):1873–1880. [PubMed] [Google Scholar]
- Ogilvie B. M., Jones V. E. Parasitological review. Nippostrongylus brasiliensis: a review of immunity and host-parasite relationship in the rat. Exp Parasitol. 1971 Feb;29(1):138–177. doi: 10.1016/0014-4894(71)90021-x. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander B., Cardell S., Möller E. Interleukin 4 and interferon gamma production in restimulated CD4+ and CD8+ cells indicates memory type responsiveness. Scand J Immunol. 1991 Mar;33(3):287–296. doi: 10.1111/j.1365-3083.1991.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Le Gros G., Ben-Sasson S. Z., Urban J., Jr, Finkelman F. D., Paul W. E. Increased frequency of interleukin 4-producing T cells as a result of polyclonal priming. Use of a single-cell assay to detect interleukin 4-producing cells. Eur J Immunol. 1991 May;21(5):1241–1247. doi: 10.1002/eji.1830210522. [DOI] [PubMed] [Google Scholar]
- Sher A., Fiorentino D., Caspar P., Pearce E., Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991 Oct 15;147(8):2713–2716. [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Stephan U., König W. Effect of antigens from Nippostrongylus brasiliensis and cytokines on the ongoing IgE synthesis in vitro. Int Arch Allergy Immunol. 1992;98(4):299–307. doi: 10.1159/000236202. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Stromberg B. E. Potentiation of the reaginic (IgE) antibody response to ovalbumin in the guinea pig with a soluble metabolic product from Ascaris suum. J Immunol. 1980 Aug;125(2):833–836. [PubMed] [Google Scholar]
- Uchikawa R., Yamada M., Matsuda S., Arizono N. IgE antibody responses induced by transplantation of the nematode Nippostrongylus brasiliensis in rats: a possible role of nematode excretory-secretory product in IgE production. Immunology. 1993 Dec;80(4):541–545. [PMC free article] [PubMed] [Google Scholar]
- Urban J. F., Jr, Katona I. M., Paul W. E., Finkelman F. D. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Kobayashi A. IgE antibody-forming cells in rats infected with Nippostrongylus brasiliensis and immunized with antigens. Cell Immunol. 1988 Sep;115(2):460–470. doi: 10.1016/0008-8749(88)90198-0. [DOI] [PubMed] [Google Scholar]
- Yamada M., Nakazawa M., Arizono N. IgE and IgG2a antibody responses are induced by different antigen groups of the nematode Nippostrongylus brasiliensis in rats. Immunology. 1993 Feb;78(2):298–302. [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Nakazawa M., Kamata I., Arizono N. Low-level infection with the nematode Nippostrongylus brasiliensis induces significant and sustained specific and non-specific IgE antibody responses in rats. Immunology. 1992 Jan;75(1):36–40. [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Nakazawa M., Matsumoto Y., Arizono N. IgE antibody production in rats against multiple components of excretory-secretory products of the nematode Nippostrongylus brasiliensis. Immunology. 1991 Jan;72(1):104–108. [PMC free article] [PubMed] [Google Scholar]


