Abstract
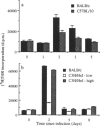
Acute, sublethal infection of mice with murine cytomegalovirus (MCMV) resulted in up to 80% decreases in the number of cells recoverable from the bone marrow, and a decrease in peripheral blood leucocyte counts during the first week of infection. Depopulation of the leucopoietic areas of the marrow was evident from examination of histological sections. The severity of bone marrow atrophy in MCMV-infected mice of different strains correlated with previously described genetically determined sensitivity to MCMV disease. Although the phenomenon only occurred when mice were inoculated with infectious virus preparations, fewer than one in 10(5) marrow cells were productively infected, suggesting that atrophy was not due to lytic infection of large numbers of bone marrow cells. Interestingly, increases in serum colony-stimulating activity were observed and these were proportional to the severity of bone marrow atrophy. After MCMV infection, we observed increases in the proportions of cells expressing some B-cell and myeloid cell markers and a decrease in the proportion of cells expressing an erythroid cell marker. There was no change in the frequency of marrow cells expressing mature T-cell markers. The numbers of myeloid lineage-committed progenitor cells (GM-CFU) in the marrow decreased 10- to 20-fold in BALB/c nu/+ mice, while there was a threefold decrease in their numbers in BALB/c nu/nu mice. In addition, increases in serum colony-stimulating activity were greater in BALB/c nu/+ mice than in BALB/c nu/nu mice. Our results suggest that growth factors produced after MCMV infection may accelerate the maturation and migration of cells from the marrow to sites of virus replication and inflammation, thus accounting for the depletion in numbers of marrow cells observed soon after MCMV infection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. E., Shellam G. R. Genetic control of murine cytomegalovirus infection: virus titres in resistant and susceptible strains of mice. Arch Virol. 1984;81(1-2):139–150. doi: 10.1007/BF01309303. [DOI] [PubMed] [Google Scholar]
- Apperley J. F., Dowding C., Hibbin J., Buiter J., Matutes E., Sissons P. J., Gordon M., Goldman J. M. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol. 1989 Jan;17(1):38–45. [PubMed] [Google Scholar]
- Bale J. F., Jr, O'Neil M. E. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J Virol. 1989 Jun;63(6):2667–2673. doi: 10.1128/jvi.63.6.2667-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale J. F., Jr, O'Neil M. E., Giller R., Perlman S., Koszinowski U. Murine cytomegalovirus genomic material in marrow cells: relation to altered leukocyte counts during sublethal infection of mice. J Infect Dis. 1987 Feb;155(2):207–212. doi: 10.1093/infdis/155.2.207. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Bartholomaeus W. N., O'Donoghue H., Foti D., Lawson C. M., Shellam G. R., Reed W. D. Multiple autoantibodies following cytomegalovirus infection: virus distribution and specificity of autoantibodies. Immunology. 1988 Jul;64(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Booth T. W., Scalzo A. A., Carrello C., Lyons P. A., Farrell H. E., Singleton G. R., Shellam G. R. Molecular and biological characterization of new strains of murine cytomegalovirus isolated from wild mice. Arch Virol. 1993;132(1-2):209–220. doi: 10.1007/BF01309855. [DOI] [PubMed] [Google Scholar]
- Busch F. W., Mutter W., Koszinowski U. H., Reddehase M. J. Rescue of myeloid lineage-committed preprogenitor cells from cytomegalovirus-infected bone marrow stroma. J Virol. 1991 Feb;65(2):981–984. doi: 10.1128/jvi.65.2.981-984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Li J. K., Falletta J. M., Wagner J. L., Lang D. J. Murine cytomegalovirus infection: hematological, morphological, and functional study of lymphoid cells. Infect Immun. 1981 Jul;33(1):239–249. doi: 10.1128/iai.33.1.239-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsele H., Steidle M., Vallbracht A., Saal J. G., Ehninger G., Müller C. A. Early occurrence of human cytomegalovirus infection after bone marrow transplantation as demonstrated by the polymerase chain reaction technique. Blood. 1991 Mar 1;77(5):1104–1110. [PubMed] [Google Scholar]
- Fiala M., Kattlove H. Letter: Cytomegalovirus mononucleosis with severe thrombocytopenia. Ann Intern Med. 1973 Sep;79(3):450–451. doi: 10.7326/0003-4819-79-3-450. [DOI] [PubMed] [Google Scholar]
- Frederickson G. G., Basch R. S. L3T4 antigen expression by hemopoietic precursor cells. J Exp Med. 1989 Apr 1;169(4):1473–1478. doi: 10.1084/jem.169.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Mackenzie J. S., Stanley N. F. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect Immun. 1981 Apr;32(1):277–286. doi: 10.1128/iai.32.1.277-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., Trapman J., Allan J. E., Shellam G. R., Melief C. J. Evidence for a protective role of interferon in resistance to murine cytomegalovirus and its control by non-H-2-linked genes. Infect Immun. 1982 Jul;37(1):143–150. doi: 10.1128/iai.37.1.143-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. The lymphocyte in infections with Epstein-Barr virus and cytomegalovirus. J Infect Dis. 1981 Jun;143(6):857–862. doi: 10.1093/infdis/143.6.857. [DOI] [PubMed] [Google Scholar]
- Horak H., Turner A. R., Shaw A. R., Yau O. W. Stimulation of [3H]thymidine uptake in mouse marrow by granulocyte-macrophage colony stimulating factor from mouse lung conditioned medium. J Immunol Methods. 1983 Jan 28;56(2):253–260. doi: 10.1016/0022-1759(83)90417-9. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Walker D. G., Altamirano M. Analysis in vitro of two biologically distinct strains of murine cytomegalovirus. Arch Virol. 1988;102(3-4):289–295. doi: 10.1007/BF01310834. [DOI] [PubMed] [Google Scholar]
- Jordan M. C., Rousseau W., Stewart J. A., Noble G. R., Chin T. D. Spontaneous cytomegalovirus mononucleosis. Clinical and laboratory observations in nine cases. Ann Intern Med. 1973 Aug;79(2):153–160. doi: 10.7326/0003-4819-79-2-153. [DOI] [PubMed] [Google Scholar]
- Koszinowski U. H., Del Val M., Reddehase M. J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., Grundy J. E., Shellam G. R. Delayed-type hypersensitivity responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1987 Sep;68(Pt 9):2379–2388. doi: 10.1099/0022-1317-68-9-2379. [DOI] [PubMed] [Google Scholar]
- Lönnqvist B., Ringdén O., Wahren B., Gahrton G., Lundgren G. Cytomegalovirus infection associated with and preceding chronic graft-versus-host disease. Transplantation. 1984 Nov;38(5):465–468. doi: 10.1097/00007890-198411000-00004. [DOI] [PubMed] [Google Scholar]
- Martin D. C., Katzenstein D. A., Yu G. S., Jordan M. C. Cytomegalovirus viremia detected by molecular hybridization and electron microscopy. Ann Intern Med. 1984 Feb;100(2):222–225. doi: 10.7326/0003-4819-100-2-222. [DOI] [PubMed] [Google Scholar]
- Mutter W., Reddehase M. J., Busch F. W., Bühring H. J., Koszinowski U. H. Failure in generating hemopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med. 1988 May 1;167(5):1645–1658. doi: 10.1084/jem.167.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Gnann J. W., Jr, Ghazal P. Regulation and tissue-specific expression of human cytomegalovirus. Curr Top Microbiol Immunol. 1990;154:75–100. doi: 10.1007/978-3-642-74980-3_4. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Shahidi N. T. Thrombocytopenia in murine cytomegalovirus infection. J Lab Clin Med. 1973 Jan;81(1):53–63. [PubMed] [Google Scholar]
- Peterson P. K., Balfour H. H., Jr, Marker S. C., Fryd D. S., Howard R. J., Simmons R. L. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine (Baltimore) 1980 Jul;59(4):283–300. [PubMed] [Google Scholar]
- Petursson S. R., Chervenick P. A., Wu B. Megakaryocytopoiesis and granulopoiesis after murine cytomegalovirus infection. J Lab Clin Med. 1984 Sep;104(3):381–390. [PubMed] [Google Scholar]
- Price P., Gibbons A. E., Shellam G. R. H-2 class I loci determine sensitivity to MCMV in macrophages and fibroblasts. Immunogenetics. 1990;32(1):20–26. doi: 10.1007/BF01787324. [DOI] [PubMed] [Google Scholar]
- Price P., Olver S. D., Gibbons A. E., Shellam G. R. B-cell activation following murine cytomegalovirus infection: implications for autoimmunity. Immunology. 1993 Jan;78(1):14–21. [PMC free article] [PubMed] [Google Scholar]
- Price P., Olver S. D., Gibbons A. E., Teo H. K., Shellam G. R. Characterization of thymic involution induced by murine cytomegalovirus infection. Immunol Cell Biol. 1993 Jun;71(Pt 3):155–165. doi: 10.1038/icb.1993.18. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Black P. H., Hirsch M. S. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J Infect Dis. 1977 Nov;136(5):667–678. doi: 10.1093/infdis/136.5.667. [DOI] [PubMed] [Google Scholar]
- Scalzo A. A., Fitzgerald N. A., Simmons A., La Vista A. B., Shellam G. R. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J Exp Med. 1990 May 1;171(5):1469–1483. doi: 10.1084/jem.171.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo A. A., Fitzgerald N. A., Wallace C. R., Gibbons A. E., Smart Y. C., Burton R. C., Shellam G. R. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J Immunol. 1992 Jul 15;149(2):581–589. [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Simmons P., Kaushansky K., Torok-Storb B. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1386–1390. doi: 10.1073/pnas.87.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Van Zee K. J., Fischer E., Hawes A. S., Hébert C. A., Terrell T. G., Baker J. B., Lowry S. F., Moldawer L. L. Effects of intravenous IL-8 administration in nonhuman primates. J Immunol. 1992 Mar 15;148(6):1746–1752. [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962 Jun 14;266:1233–1244. doi: 10.1056/NEJM196206142662401. [DOI] [PubMed] [Google Scholar]
- Wykes M. N., Shellam G. R., McCluskey J., Kast W. M., Dallas P. B., Price P. Murine cytomegalovirus interacts with major histocompatibility complex class I molecules to establish cellular infection. J Virol. 1993 Jul;67(7):4182–4189. doi: 10.1128/jvi.67.7.4182-4189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager A. S., Palumbo P. E., Malachowski N., Ariagno R. L., Stevenson D. K. Sequelae of maternally derived cytomegalovirus infections in premature infants. J Pediatr. 1983 Jun;102(6):918–922. doi: 10.1016/s0022-3476(83)80025-0. [DOI] [PubMed] [Google Scholar]