Abstract
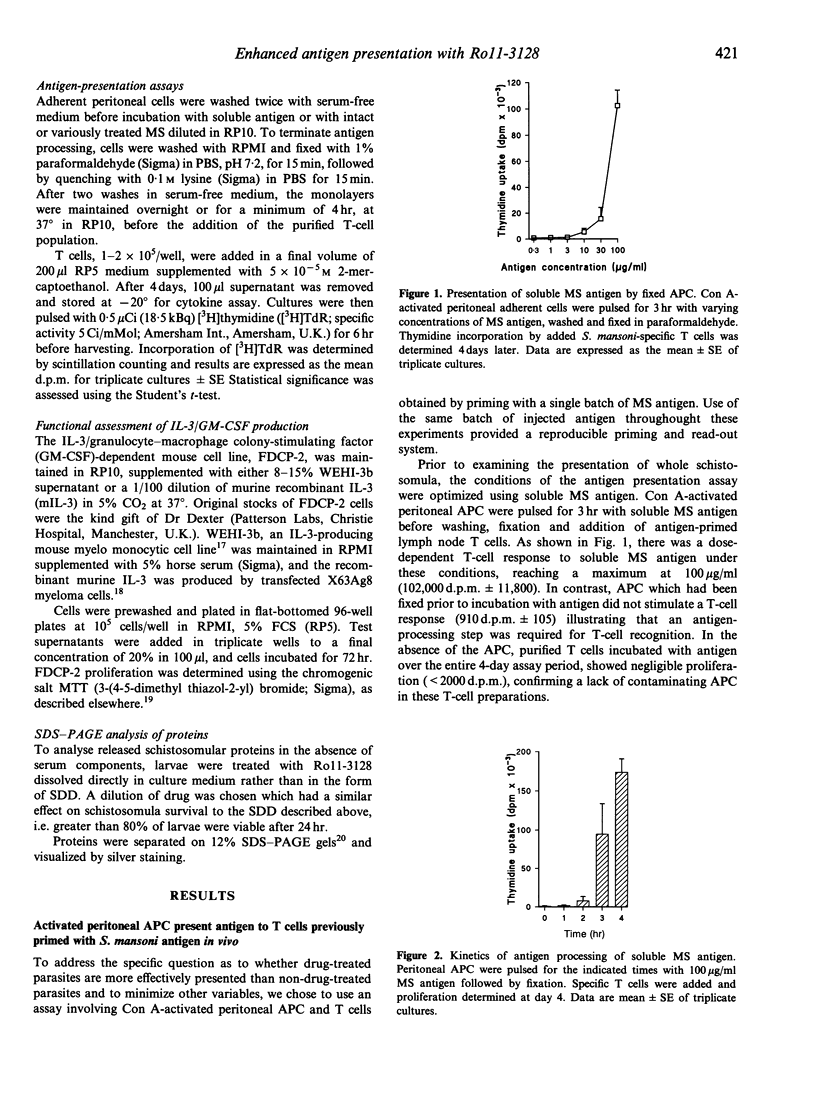
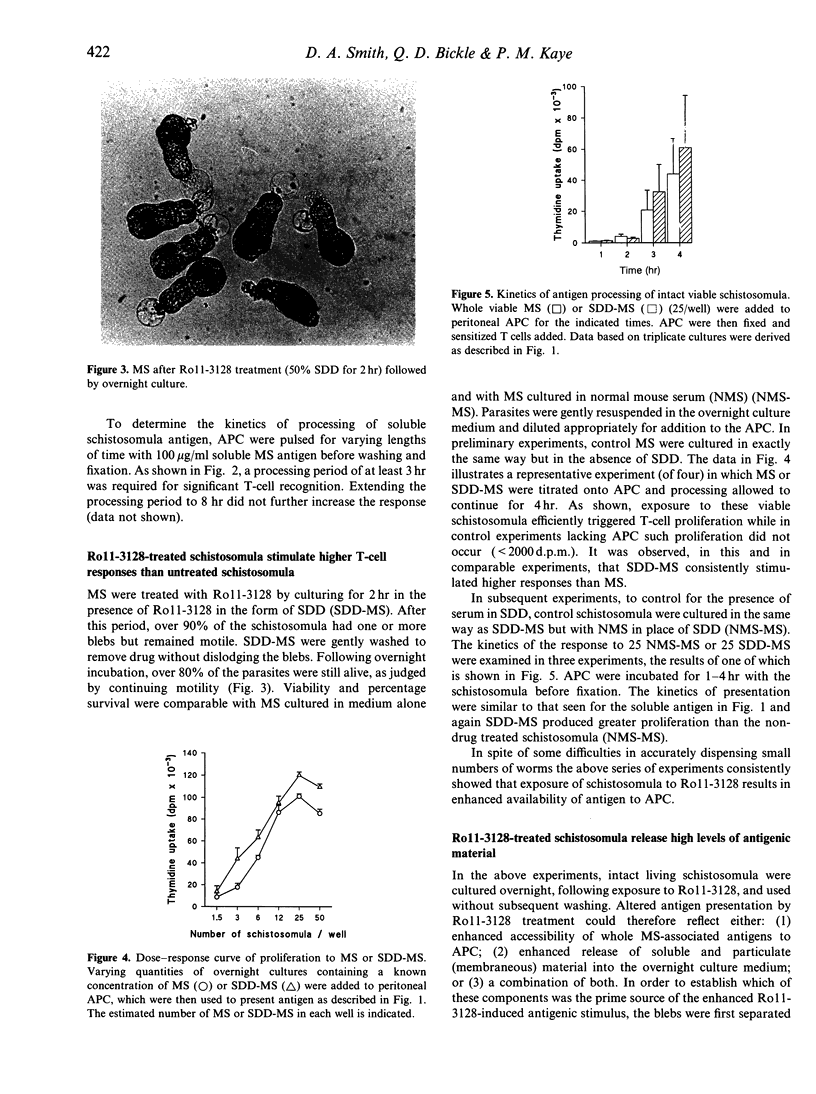
Treatment of mice with the benzodiazepine derivative Ro11-3128 1-2 days post-infection with Schistosoma mansoni leads to arrest of virtually all schistosomula at the skin stage, and results in the development of protective immunity to challenge infection. A characteristic feature of Ro11-3128 treatment in vitro is the formation of exudates and membranous blebs at the schistosomular surface; other drugs tested, such as Ro15-5458 and oxamniquine which are also effective against the skin stages but relatively ineffective in inducing protection, do not induce this reaction. Here, we have examined whether such in vitro treatment causes enhanced presentation of schistosomular antigens by host antigen-presenting cells (APC) using an in vitro assay with activated peritoneal adherent cells as APC and T cells from S. mansoni antigen-sensitized mice. We have shown that viable mechanically transformed schistosomula (MS) can be processed and presented with similar kinetics to soluble antigen. However, in vitro drug treatment leads to enhanced presentation of MS. Experiments in which membranous blebs and antigen released by Ro11-3128-treated parasites during in vitro culture were separated from the remaining intact schistosomula, demonstrated significant stimulatory activity in the soluble and particulate-released antigen fractions. Filtration, antigen transfer experiments and SDS-PAGE analysis of the released material further suggested that most of the activity resided in the particulate fraction. Thus, quantitative and qualitative changes to antigen presentation by Ro11-3128 treatment early after infection may underlie the immunoprotective efficacy of Ro11-3128-abbreviated infections.
Full text
PDF

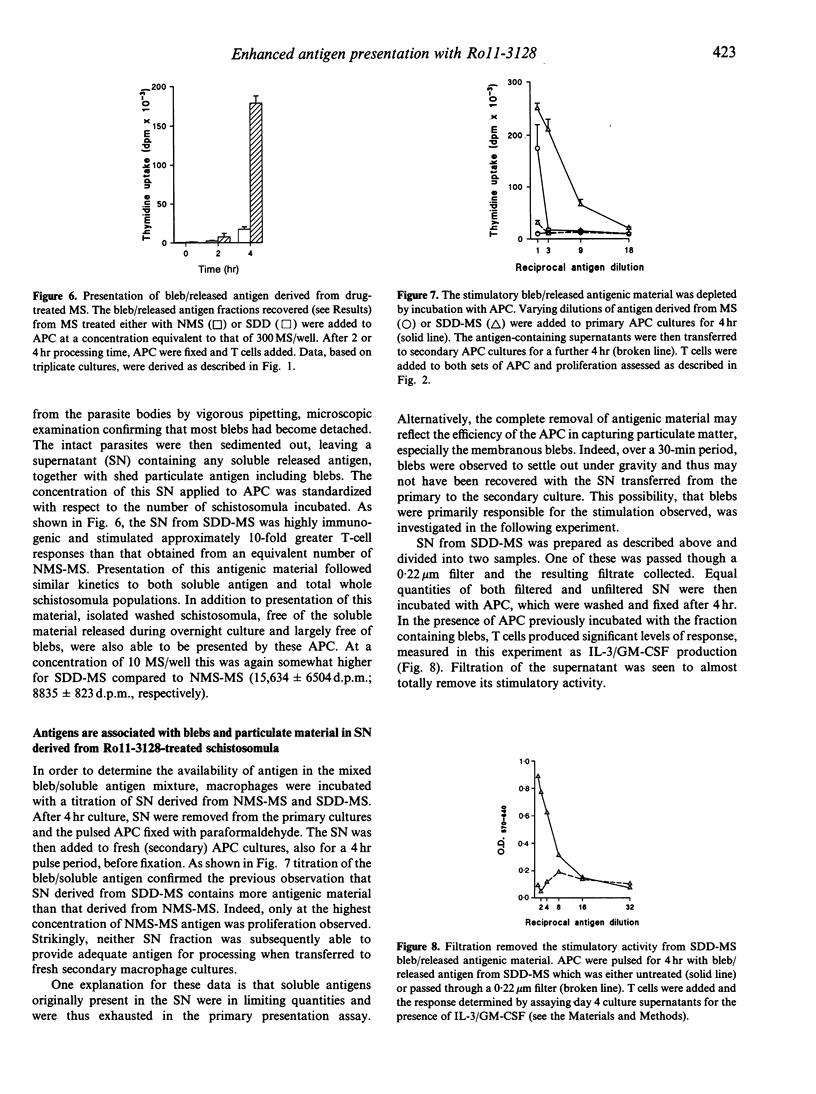



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auriault C., Damonneville M., Verwaerde C., Pierce R., Joseph M., Capron M., Capron A. Rat IgE directed against schistosomula-released products is cytotoxic for Schistosoma mansoni schistosomula in vitro. Eur J Immunol. 1984 Feb;14(2):132–138. doi: 10.1002/eji.1830140206. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Bickle Q. D., Andrews B. J. Resistance following drug attenuation (Ro 11-3128 or oxamniquine) of early Schistosoma mansoni infections in mice. Parasitology. 1985 Apr;90(Pt 2):325–338. doi: 10.1017/s0031182000051027. [DOI] [PubMed] [Google Scholar]
- Bickle Q. D., Dobinson T., James E. R. The effects of gamma-irradiation on migration and survival of Schistosoma mansoni schistosomula in mice. Parasitology. 1979 Oct;79(2):223–230. doi: 10.1017/s0031182000053300. [DOI] [PubMed] [Google Scholar]
- Caulada-Benedetti Z., al-Zamel F., Sher A., James S. Comparison of Th1- and Th2-associated immune reactivities stimulated by single versus multiple vaccination of mice with irradiated Schistosoma mansoni cercariae. J Immunol. 1991 Mar 1;146(5):1655–1660. [PubMed] [Google Scholar]
- Collins H. L., Bancroft G. J. Encapsulation of Cryptococcus neoformans impairs antigen-specific T-cell responses. Infect Immun. 1991 Nov;59(11):3883–3888. doi: 10.1128/iai.59.11.3883-3888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudot-Crozel V., Caillol D., Djabali M., Dessein A. J. The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kD glyceraldehyde-3P-dehydrogenase. J Exp Med. 1989 Dec 1;170(6):2065–2080. doi: 10.1084/jem.170.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harn D. A., Gu W., Oligino L. D., Mitsuyama M., Gebremichael A., Richter D. A protective monoclonal antibody specifically recognizes and alters the catalytic activity of schistosome triose-phosphate isomerase. J Immunol. 1992 Jan 15;148(2):562–567. [PubMed] [Google Scholar]
- James E. R., Taylor M. G. Transformation of cercariae to schistosomula: a quantitative comparison of transformation techniques and of infectivity by different injection routes of the organisms produced. J Helminthol. 1976 Dec;50(4):223–233. doi: 10.1017/s0022149x0002664x. [DOI] [PubMed] [Google Scholar]
- James S. L., Skamene E., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. V. Variation in macrophage schistosomulacidal and tumoricidal activities among mouse strains and correlation with resistance to reinfection. J Immunol. 1983 Aug;131(2):948–953. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kaye P. M. Antigen presentation and the response to parasitic infection. Parasitol Today. 1987 Oct;3(10):293–299. doi: 10.1016/0169-4758(87)90186-4. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Chain B. M., Feldmann M. Nonphagocytic dendritic cells are effective accessory cells for anti-mycobacterial responses in vitro. J Immunol. 1985 Mar;134(3):1930–1934. [PubMed] [Google Scholar]
- Kaye P. M., Coburn C., McCrossan M., Beverley S. M. Antigens targeted to the Leishmania phagolysosome are processed for CD4+ T cell recognition. Eur J Immunol. 1993 Sep;23(9):2311–2319. doi: 10.1002/eji.1830230939. [DOI] [PubMed] [Google Scholar]
- Kelly E. A., Colley D. G. In vivo effects of monoclonal anti-L3T4 antibody on immune responsiveness of mice infected with Schistosoma mansoni. Reduction of irradiated cercariae-induced resistance. J Immunol. 1988 Apr 15;140(8):2737–2745. [PubMed] [Google Scholar]
- Kusel J. R., Gazzinelli G., Colley D. G., de Souza C. P., Cordeiro M. N. The formation of surface membrane vesicles from schistosomula of Schistosoma mansoni. Parasitology. 1984 Dec;89(Pt 3):483–494. doi: 10.1017/s0031182000056717. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang T., Kaye P. M. Presentation of Leishmania donovani promastigotes occurs via a brefeldin A-sensitive pathway. Eur J Immunol. 1991 Oct;21(10):2407–2413. doi: 10.1002/eji.1830211017. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Hapel A. J., Ihle J. N. Constitutive production of a unique lymphokine (IL 3) by the WEHI-3 cell line. J Immunol. 1982 Jun;128(6):2393–2398. [PubMed] [Google Scholar]
- Mitchell G. F., Wright M. D., Wood S. M., Tiu W. U. Further studies on variable resistance of 129/J and C57BL/6 mice to infection with Schistosoma japonicum and Schistosoma mansoni. Parasite Immunol. 1990 Nov;12(6):559–567. doi: 10.1111/j.1365-3024.1990.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Mountford A. P., Coulson P. S., Saunders N., Wilson R. A. Characteristics of protective immunity in mice induced by drug-attenuated larvae of Schistosoma mansoni. Antigen localization and antibody responses. J Immunol. 1989 Aug 1;143(3):989–995. [PubMed] [Google Scholar]
- Pearce E. J., Basch P. F., Sher A. Evidence that the reduced surface antigenicity of developing Schistosoma mansoni schistosomula is due to antigen shedding rather than host molecule acquisition. Parasite Immunol. 1986 Jan;8(1):79–94. doi: 10.1111/j.1365-3024.1986.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. J., Sher A. Three major surface antigens of Schistosoma mansoni are linked to the membrane by glycosylphosphatidylinositol. J Immunol. 1989 Feb 1;142(3):979–984. [PubMed] [Google Scholar]
- Phillips S. M., Lin J. J., Galal N., Tung A. S., Linette G. P., Perrin P. J. Resistance in murine schistosomiasis is contingent on activated IL-2 receptor-bearing L3T4+ lymphocytes, negatively regulated by Lyt-2+ cells, and uninfluenced by the presence of IL-4. J Immunol. 1991 Feb 15;146(4):1335–1340. [PubMed] [Google Scholar]
- Sauma S. Y., Tanaka T. M., Strand M. Selective release of a glycosylphosphatidylinositol-anchored antigen from the surface of Schistosoma mansoni. Mol Biochem Parasitol. 1991 May;46(1):73–80. doi: 10.1016/0166-6851(91)90200-p. [DOI] [PubMed] [Google Scholar]
- Simpson A. J., Payares G., Walker T., Knight M., Smithers S. R. The modulation of expression of polypeptide surface antigens on developing schistosomula of Schistosoma mansoni. J Immunol. 1984 Nov;133(5):2725–2730. [PubMed] [Google Scholar]
- Smythies L. E., Coulson P. S., Wilson R. A. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992 Dec 1;149(11):3654–3658. [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. B., Vidal A., Torpier G., Meyer D. J., Roitsch C., Balloul J. M., Southan C., Sondermeyer P., Pemble S., Lecocq J. P. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 1988 Feb;7(2):465–472. doi: 10.1002/j.1460-2075.1988.tb02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Cerottini J. C. Antigen presentation. FASEB J. 1989 Nov;3(13):2496–2502. doi: 10.1096/fasebj.3.13.2572499. [DOI] [PubMed] [Google Scholar]
- Verwaerde C., Joseph M., Capron M., Pierce R. J., Damonneville M., Velge F., Auriault C., Capron A. Functional properties of a rat monoclonal IgE antibody specific for Schistosoma mansoni. J Immunol. 1987 Jun 15;138(12):4441–4446. [PubMed] [Google Scholar]
- Vignali D. A., Crocker P., Bickle Q. D., Cobbold S., Waldmann H., Taylor M. G. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology. 1989 Aug;67(4):466–472. [PMC free article] [PubMed] [Google Scholar]