Abstract
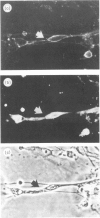
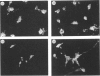
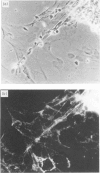
The expression of CD59, a complement regulator of the formation and function of the terminal cytolytic membrane attack complex, was studied in human normal nervous tissue by immunohistochemical markers using two monoclonal antibodies 1F5 and MEM43. CD59 was present on Schwann cells, neurons and endothelial cells in the peripheral nervous system (PNS), and on Schwann cells in culture. In the central nervous system (CNS) CD59 was found predominantly on endothelial cells. There was also a diffuse staining of white and grey matter of the spinal cord and brain, presumably of microglia, oligodendrocytes, astrocytes and neurons, as these cells were CD59 positive in culture. Furthermore, CD59 was detected in the cerebrospinal fluid (CSF) of healthy individuals. CD59 in the PNS and CNS was glycosyl-phosphatidylinositol linked and had a molecular weight of 19,000-25,000. The presence of CD59 on various cells of the nervous system and in the CSF suggests that regulation of complement activation by this protein is important in neural host defence mechanisms.
Full text
PDF

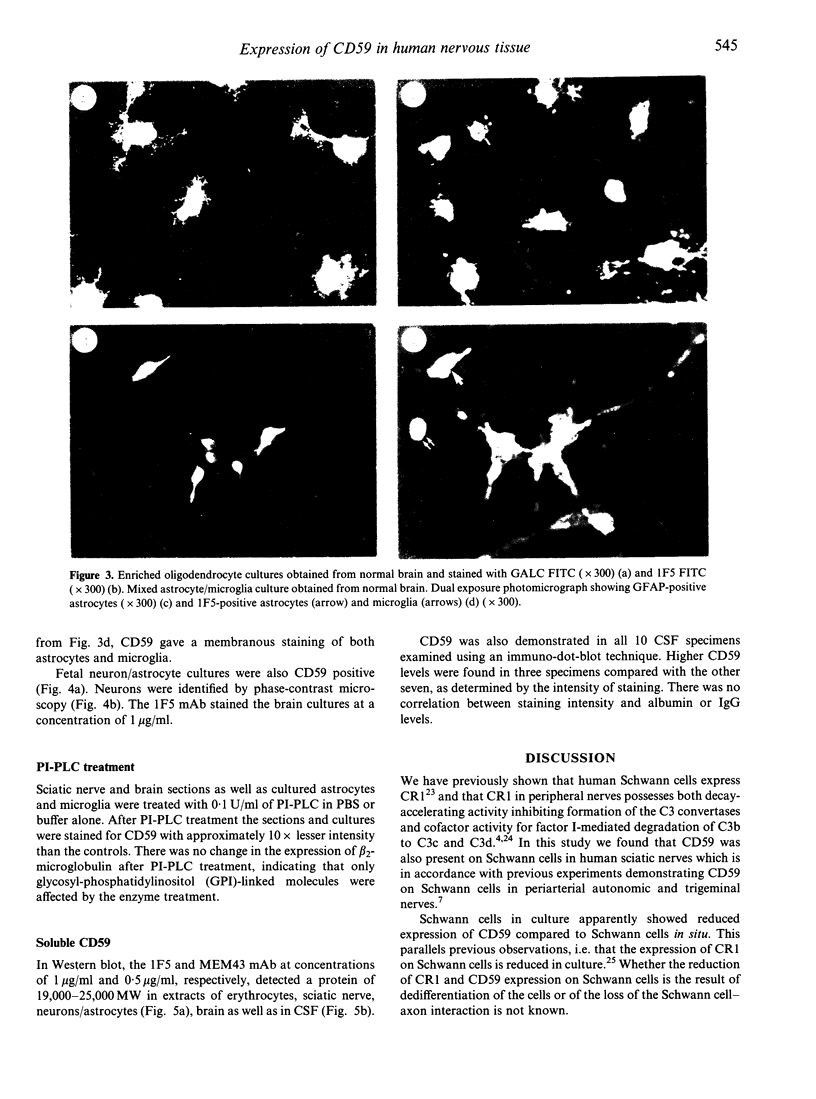


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arulanandam A. R., Moingeon P., Concino M. F., Recny M. A., Kato K., Yagita H., Koyasu S., Reinherz E. L. A soluble multimeric recombinant CD2 protein identifies CD48 as a low affinity ligand for human CD2: divergence of CD2 ligands during the evolution of humans and mice. J Exp Med. 1993 May 1;177(5):1439–1450. doi: 10.1084/jem.177.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørge L., Jensen T. S., Vedeler C. A., Ulvestad E., Kristoffersen E. K., Matre R. Soluble CD59 in pregnancy and infancy. Immunol Lett. 1993 May;36(2):233–233. doi: 10.1016/0165-2478(93)90058-a. [DOI] [PubMed] [Google Scholar]
- Compston A., Scolding N., Wren D., Noble M. The pathogenesis of demyelinating disease: insights from cell biology. Trends Neurosci. 1991 May;14(5):175–182. doi: 10.1016/0166-2236(91)90099-g. [DOI] [PubMed] [Google Scholar]
- Compston D. A., Morgan B. P., Campbell A. K., Wilkins P., Cole G., Thomas N. D., Jasani B. Immunocytochemical localization of the terminal complement complex in multiple sclerosis. Neuropathol Appl Neurobiol. 1989 Jul-Aug;15(4):307–316. doi: 10.1111/j.1365-2990.1989.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. L., Sadlon T., Hefford C., Adrian D. Expression of CD59, a regulator of the membrane attack complex of complement, on human astrocytes. Brain Res Mol Brain Res. 1993 Jun;18(4):335–338. doi: 10.1016/0169-328x(93)90098-a. [DOI] [PubMed] [Google Scholar]
- Hahn W. C., Menu E., Bothwell A. L., Sims P. J., Bierer B. E. Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science. 1992 Jun 26;256(5065):1805–1807. doi: 10.1126/science.1377404. [DOI] [PubMed] [Google Scholar]
- Henneberg A., Mayle D. M., Kornhuber H. H. Antibodies to brain tissue in sera of patients with chronic progressive multiple sclerosis. J Neuroimmunol. 1991 Nov;34(2-3):223–227. doi: 10.1016/0165-5728(91)90133-r. [DOI] [PubMed] [Google Scholar]
- Koski C. L., Sanders M. E., Swoveland P. T., Lawley T. J., Shin M. L., Frank M. M., Joiner K. A. Activation of terminal components of complement in patients with Guillain-Barré syndrome and other demyelinating neuropathies. J Clin Invest. 1987 Nov;80(5):1492–1497. doi: 10.1172/JCI113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski C. L., Vanguri P., Shin M. L. Activation of the alternative pathway of complement by human peripheral nerve myelin. J Immunol. 1985 Mar;134(3):1810–1814. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Walker D. G., Akiyama H., Kawamata T., Guan A. L., Parker C. J., Okada N., McGeer E. G. Detection of the membrane inhibitor of reactive lysis (CD59) in diseased neurons of Alzheimer brain. Brain Res. 1991 Mar 29;544(2):315–319. doi: 10.1016/0006-8993(91)90071-3. [DOI] [PubMed] [Google Scholar]
- Meri S., Waldmann H., Lachmann P. J. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991 Nov;65(5):532–537. [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nose M., Katoh M., Okada N., Kyogoku M., Okada H. Tissue distribution of HRF20, a novel factor preventing the membrane attack of homologous complement, and its predominant expression on endothelial cells in vivo. Immunology. 1990 Jun;70(2):145–149. [PMC free article] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Rollins S. A., Zhao J., Ninomiya H., Sims P. J. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991 Apr 1;146(7):2345–2351. [PubMed] [Google Scholar]
- Sanders M. E., Koski C. L., Robbins D., Shin M. L., Frank M. M., Joiner K. A. Activated terminal complement in cerebrospinal fluid in Guillain-Barré syndrome and multiple sclerosis. J Immunol. 1986 Jun 15;136(12):4456–4459. [PubMed] [Google Scholar]
- Scarpini E., Kreider B. Q., Lisak R. P., Pleasure D. E. Establishment of Schwann cell cultures from adult rat peripheral nerves. Exp Neurol. 1988 Nov;102(2):167–176. doi: 10.1016/0014-4886(88)90090-8. [DOI] [PubMed] [Google Scholar]
- Schönermark S., Rauterberg E. W., Shin M. L., Löke S., Roelcke D., Hänsch G. M. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986 Mar 1;136(5):1772–1776. [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Rollins S. A., Wiedmer T. Regulatory control of complement on blood platelets. Modulation of platelet procoagulant responses by a membrane inhibitor of the C5b-9 complex. J Biol Chem. 1989 Nov 15;264(32):19228–19235. [PubMed] [Google Scholar]
- Stefanová I., Hilgert I., Kristofová H., Brown R., Low M. G., Horejsí V. Characterization of a broadly expressed human leucocyte surface antigen MEM-43 anchored in membrane through phosphatidylinositol. Mol Immunol. 1989 Feb;26(2):153–161. doi: 10.1016/0161-5890(89)90097-7. [DOI] [PubMed] [Google Scholar]
- Stott D. I. Immunoblotting and dot blotting. J Immunol Methods. 1989 May 12;119(2):153–187. doi: 10.1016/0022-1759(89)90394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanguri P., Koski C. L., Silverman B., Shin M. L. Complement activation by isolated myelin: activation of the classical pathway in the absence of myelin-specific antibodies. Proc Natl Acad Sci U S A. 1982 May;79(10):3290–3294. doi: 10.1073/pnas.79.10.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedeler C. A., Matre R. Complement receptors CR1 on human peripheral nerve fibres. J Neuroimmunol. 1988 Mar;17(4):315–322. doi: 10.1016/0165-5728(88)90122-1. [DOI] [PubMed] [Google Scholar]
- Vedeler C. A., Matre R., Fischer E. Isolation and characterization of complement receptors CR1 from human peripheral nerves. J Neuroimmunol. 1989 Aug;23(3):215–221. doi: 10.1016/0165-5728(89)90053-2. [DOI] [PubMed] [Google Scholar]
- Vedeler C. A., Matre R., Nyland H. Class and IgG subclass distribution of antibodies against peripheral nerve myelin in sera from patients with inflammatory demyelinating polyradiculoneuropathy. Acta Neurol Scand. 1988 Nov;78(5):401–407. doi: 10.1111/j.1600-0404.1988.tb03676.x. [DOI] [PubMed] [Google Scholar]
- Vedeler C. A., Matre R. Peripheral nerve CR1 express in situ cofactor activity for degradation of C3b. J Neuroimmunol. 1990 Jan;26(1):51–56. doi: 10.1016/0165-5728(90)90119-8. [DOI] [PubMed] [Google Scholar]
- Wing M. G., Zajicek J., Seilly D. J., Compston D. A., Lachmann P. J. Oligodendrocytes lack glycolipid anchored proteins which protect them against complement lysis. Restoration of resistance to lysis by incorporation of CD59. Immunology. 1992 May;76(1):140–145. [PMC free article] [PubMed] [Google Scholar]