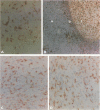
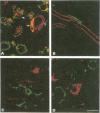
Abstract
Human leucocyte antigen (HLA) class II molecules expressed by antigen-presenting cells (APC) are major restriction elements in the interaction between APC and T cells of the CD4+ subtype. To explore the immune accessory function of cells in the central nervous system (CNS), we studied the expression of HLA-DR, -DP, and -DQ molecules on CNS cells in situ and in vitro. Reactive microglia and perivascular cells in multiple sclerosis lesions expressed all three HLA class II molecules, whereas microglia in the normal CNS expressed HLA-DR only. All three HLA class II molecules were up-regulated on cultured microglia after stimulation with interferon-gamma (IFN-gamma). Microglial stimulation of allogeneic CD4+ T cells in a mixed lymphocyte reaction (MLR) was effectively blocked using anti-HLA-DR monoclonal antibodies (mAb) but not using anti-HLA-DQ mAb. HLA class II-positive astrocytes and endothelial cells were not identified in normal or diseased CNS. Cultured astrocytes stimulated with IFN-gamma could, however, be induced to express HLA class II antigens of all subtypes, although great variability was observed between different donors. Our results indicate that although both microglia and astrocytes are capable of expressing all HLA class II subtypes in vitro, subtype expression differs between normal and pathological states in situ. Such selective expression may be associated with functional properties.
Full text
PDF


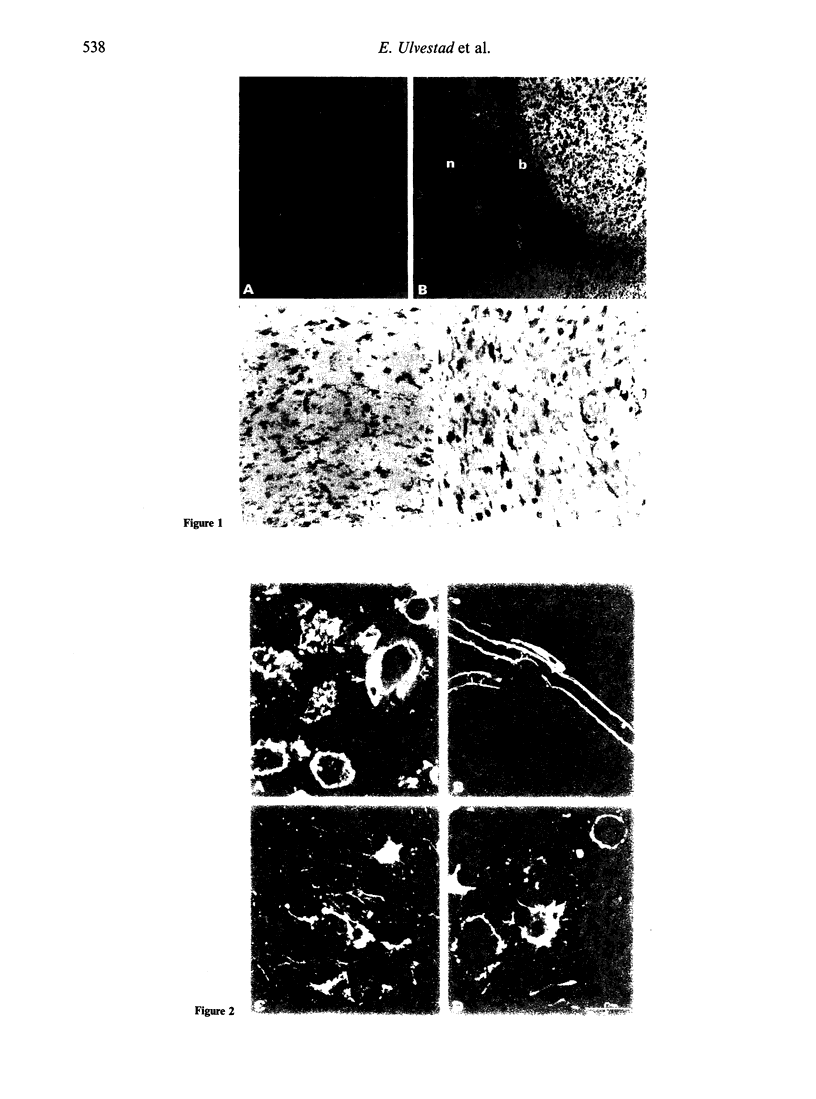
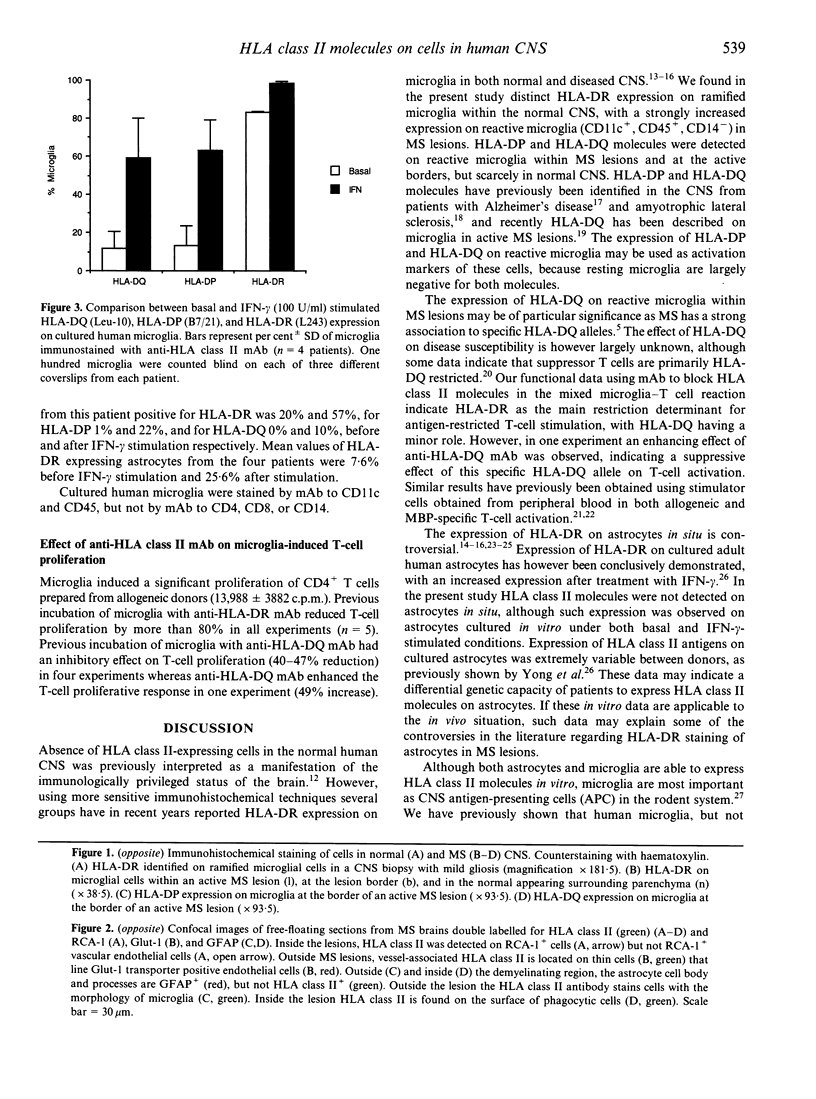


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Sansom D., Marsh S. G. What is the basis for HLA-DQ associations with autoimmune disease? Immunol Today. 1991 Aug;12(8):267–270. doi: 10.1016/0167-5699(91)90124-C. [DOI] [PubMed] [Google Scholar]
- Boyle E. A., McGeer P. L. Cellular immune response in multiple sclerosis plaques. Am J Pathol. 1990 Sep;137(3):575–584. [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Chou Y. K., Vainiene M., Whitham R., Bourdette D., Chou C. H., Hashim G., Offner H., Vandenbark A. A. Response of human T lymphocyte lines to myelin basic protein: association of dominant epitopes with HLA class II restriction molecules. J Neurosci Res. 1989 Jun;23(2):207–216. doi: 10.1002/jnr.490230211. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Estes M. L., Rudick R. A., Barnett G. H., Ransohoff R. M. Stereotactic biopsy of an active multiple sclerosis lesion. Immunocytochemical analysis and neuropathologic correlation with magnetic resonance imaging. Arch Neurol. 1990 Dec;47(12):1299–1303. doi: 10.1001/archneur.1990.00530120043008. [DOI] [PubMed] [Google Scholar]
- Fairchild P. J., Wraith D. C. Peptide-MHC interaction in autoimmunity. Curr Opin Immunol. 1992 Dec;4(6):748–753. doi: 10.1016/0952-7915(92)90056-k. [DOI] [PubMed] [Google Scholar]
- Gehrmann J., Banati R. B., Kreutzberg G. W. Microglia in the immune surveillance of the brain: human microglia constitutively express HLA-DR molecules. J Neuroimmunol. 1993 Nov-Dec;48(2):189–198. doi: 10.1016/0165-5728(93)90191-z. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Büringer D., Sparks D. L., Kreutzberg G. W. Ultrastructural location of major histocompatibility complex (MHC) class II positive perivascular cells in histologically normal human brain. J Neuropathol Exp Neurol. 1992 May;51(3):303–311. doi: 10.1097/00005072-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Hayes G. M., Woodroofe M. N., Cuzner M. L. Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci. 1987 Aug;80(1):25–37. doi: 10.1016/0022-510x(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., von Hanwehr R. I., Dinarello C. A., Mizel S. B., Hinton D., Merrill J. E. Immunoregulatory molecules and IL 2 receptors identified in multiple sclerosis brain. J Immunol. 1986 May 1;136(9):3239–3245. [PubMed] [Google Scholar]
- Huynh H. K., Dorovini-Zis K. Effects of interferon-gamma on primary cultures of human brain microvessel endothelial cells. Am J Pathol. 1993 Apr;142(4):1265–1278. [PMC free article] [PubMed] [Google Scholar]
- Joshi N., Usuku K., Hauser S. L. The T-cell response to myelin basic protein in familial multiple sclerosis: diversity of fine specificity, restricting elements, and T-cell receptor usage. Ann Neurol. 1993 Sep;34(3):385–393. doi: 10.1002/ana.410340313. [DOI] [PubMed] [Google Scholar]
- Kawamata T., Akiyama H., Yamada T., McGeer P. L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol. 1992 Mar;140(3):691–707. [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Moore G. R., Golenwsky G., Raine C. S. Multiple sclerosis: a role for astroglia in active demyelination suggested by class II MHC expression and ultrastructural study. J Neuropathol Exp Neurol. 1990 Mar;49(2):122–136. doi: 10.1097/00005072-199003000-00005. [DOI] [PubMed] [Google Scholar]
- Li H., Newcombe J., Groome N. P., Cuzner M. L. Characterization and distribution of phagocytic macrophages in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1993 Jun;19(3):214–223. doi: 10.1111/j.1365-2990.1993.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Liblau R., Tournier-Lasserve E., Maciazek J., Dumas G., Siffert O., Hashim G., Bach M. A. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991 Jun;21(6):1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- Luber-Narod J., Rogers J. Immune system associated antigens expressed by cells of the human central nervous system. Neurosci Lett. 1988 Nov 22;94(1-2):17–22. doi: 10.1016/0304-3940(88)90263-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Ohmori K., Fujiwara M. Immune regulation by brain cells in the central nervous system: microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology. 1992 Jun;76(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Myers K. J., Dougherty J. P., Ron Y. In vivo antigen presentation by both brain parenchymal cells and hematopoietically derived cells during the induction of experimental autoimmune encephalomyelitis. J Immunol. 1993 Aug 15;151(4):2252–2260. [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., Hirsch R. L., Schindler J., Johnson K. P. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987 Jul;37(7):1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Rosa F., Hatat D., Abadie A., Wallach D., Revel M., Fellous M. Differential regulation of HLA-DR mRNAs and cell surface antigens by interferon. EMBO J. 1983;2(9):1585–1589. doi: 10.1002/j.1460-2075.1983.tb01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D., Schwender S., Gregersen R., Dörries R., ter Meulen V. Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med. 1993 Apr 1;177(4):1145–1152. doi: 10.1084/jem.177.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurkland A., Rønningen K. S., Vandvik B., Thorsby E., Vartdal F. HLA-DQA1 and HLA-DQB1 genes may jointly determine susceptibility to develop multiple sclerosis. Hum Immunol. 1991 Jan;30(1):69–75. doi: 10.1016/0198-8859(91)90073-i. [DOI] [PubMed] [Google Scholar]
- Steiniger B., van der Meide P. H. Rat ependyma and microglia cells express class II MHC antigens after intravenous infusion of recombinant gamma interferon. J Neuroimmunol. 1988 Aug;19(1-2):111–118. doi: 10.1016/0165-5728(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Styren S. D., Civin W. H., Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer's disease brain. Exp Neurol. 1990 Oct;110(1):93–104. doi: 10.1016/0014-4886(90)90054-v. [DOI] [PubMed] [Google Scholar]
- Thorsby E. Structure and function of HLA molecules. Transplant Proc. 1987 Feb;19(1 Pt 1):29–35. [PubMed] [Google Scholar]
- Traugott U., Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann Neurol. 1988 Aug;24(2):243–251. doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- Uitdehaag B. M., de Groot C. J., Kreike A., van der Meide P. H., Polman C. H., Dijkstra C. D. The significance of in-situ Ia antigen expression in the pathogenesis of autoimmune central nervous system disease. J Autoimmun. 1993 Jun;6(3):323–335. doi: 10.1006/jaut.1993.1028. [DOI] [PubMed] [Google Scholar]
- Williams K., Bar-Or A., Ulvestad E., Olivier A., Antel J. P., Yong V. W. Biology of adult human microglia in culture: comparisons with peripheral blood monocytes and astrocytes. J Neuropathol Exp Neurol. 1992 Sep;51(5):538–549. doi: 10.1097/00005072-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Williams K., Jr, Ulvestad E., Cragg L., Blain M., Antel J. P. Induction of primary T cell responses by human glial cells. J Neurosci Res. 1993 Nov 1;36(4):382–390. doi: 10.1002/jnr.490360404. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]