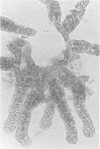
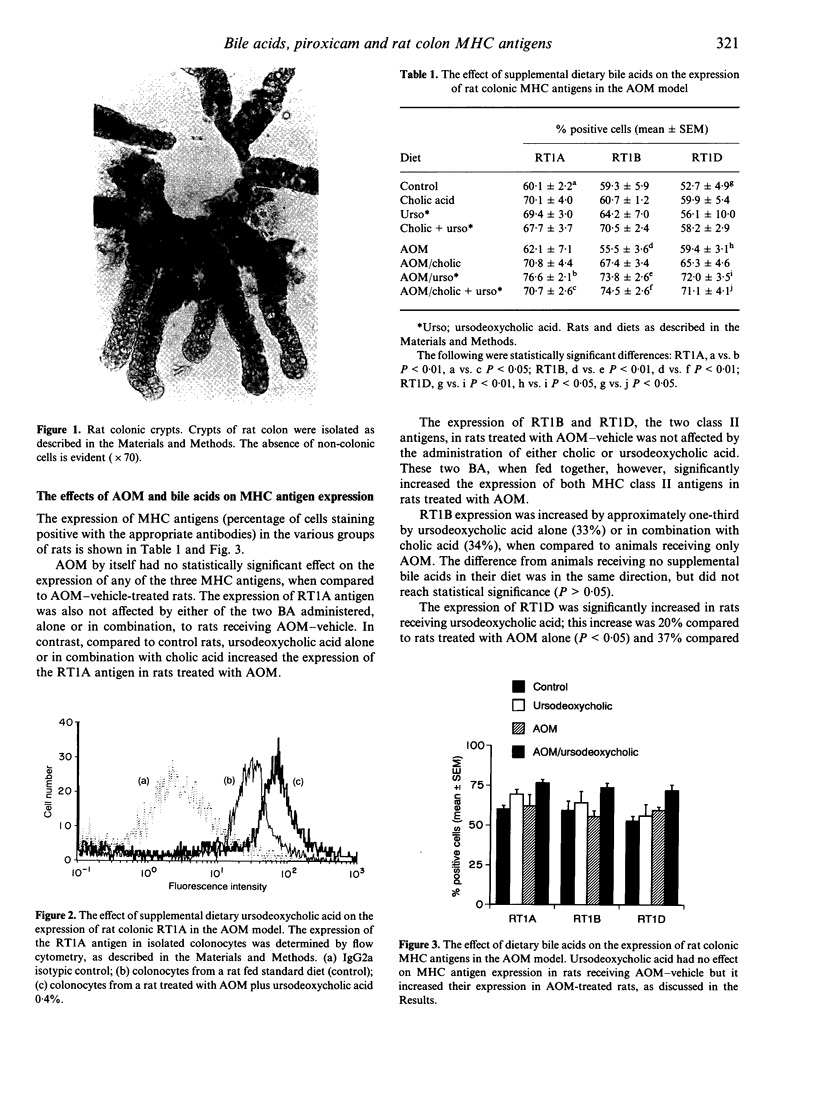
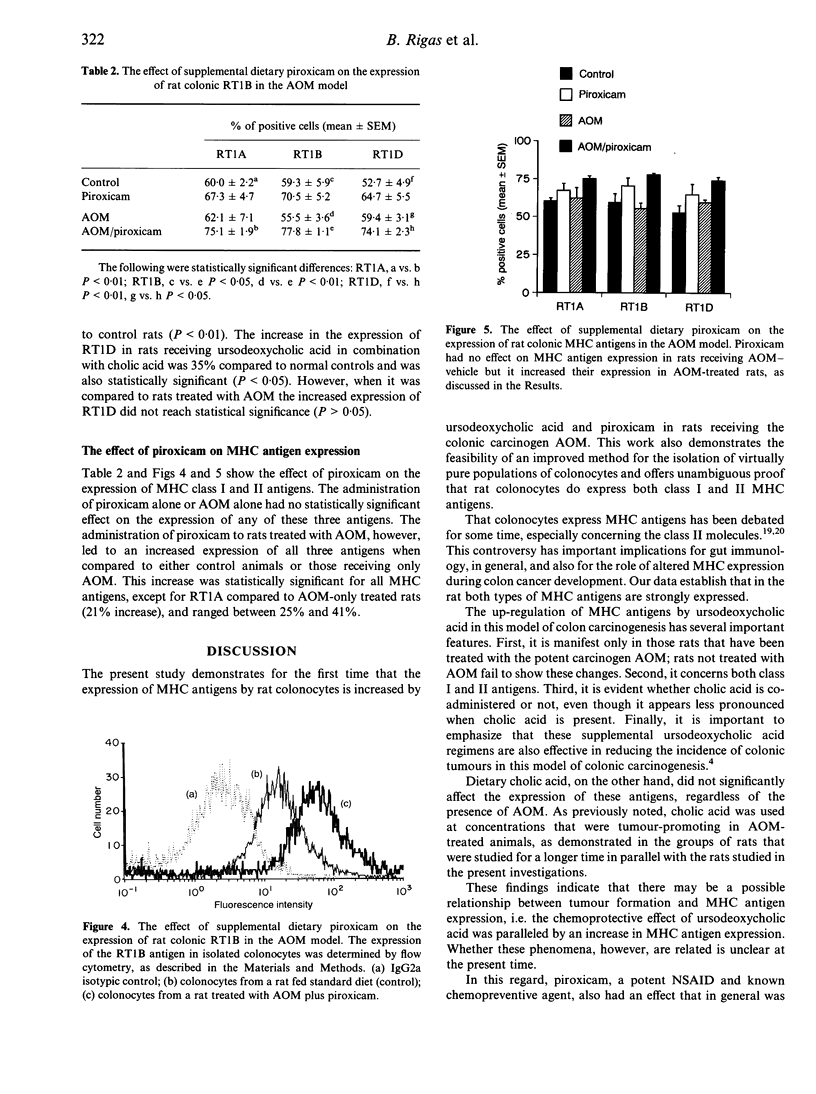
Abstract
The effect of bile acids and piroxicam on the expression of major histocompatibility complex (MHC) antigens in colonocytes was evaluated in rats treated with the colonic carcinogen azoxymethane (AOM). Male Fischer-344 rats were fed a basal diet (AIN-76) supplemented with 0.4% cholic acid, 0.4% ursodeoxycholic acid, 0.2% ursodeoxycholic acid plus 0.2% cholic acid, or 75 p.p.m. piroxicam. Rats were injected subcutaneously once a week for 2 weeks with AOM (15 mg/kg body weight/week) or vehicle, after being fed their respective diets for two weeks. The rats were killed at 16 weeks, while parallel identical groups of rats were killed at 28 weeks, and colon tumours were counted. None of the rats treated with AOM-vehicle developed tumours at 28 weeks, while in the AOM-treated rats the frequency of colonic tumours was as follows: AOM alone 50%, cholic acid 74%, ursodeoxycholic acid 17%, piroxicam 28%, ursodeoxycholic plus cholic acid 46%. The expression of RT1A, RT1B and RT1D was determined in isolated colonocytes by immune fluocytometry. Normal rat colonocytes express all three MHC antigens strongly. Neither the bile acids nor piroxicam affected MHC antigen expression in AOM-vehicle-treated rats. AOM did not effect MHC antigen expression compared to normal controls. Cholic acid had no significant effect on the expression of MHC antigens in AOM-treated rats. Ursodeoxycholic acid alone or in combination with cholic acid increased the expression of RT1A compared to normal controls, of RT1B compared to AOM-treated rats, and of RT1D compared to controls or AOM-treated rats. Piroxicam increased the expression of all three antigens compared to either control or AOM-treated rats. These findings indicate that (1) ursodeoxycholic acid and piroxicam up-regulate colonic MHC antigen expression in the AOM model of colonic carcinogenesis; (2) the colon of rats exposed to AOM responds differently than the normal colon with respect to MHC regulation; and (3) the protective effect of ursodeoxycholic acid and piroxicam on colon tumour formation seems to be paralleled by an increase in MHC antigen expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodmer W. T-cell immune responses to cancer--a new look. Hum Immunol. 1991 Apr;30(4):259–261. doi: 10.1016/0198-8859(91)90004-s. [DOI] [PubMed] [Google Scholar]
- Cheng H., Bjerknes M., Amar J. Methods for the determination of epithelial cell kinetic parameters of human colonic epithelium isolated from surgical and biopsy specimens. Gastroenterology. 1984 Jan;86(1):78–85. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Giardiello F. M., Hamilton S. R., Krush A. J., Piantadosi S., Hylind L. M., Celano P., Booker S. V., Robinson C. R., Offerhaus G. J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993 May 6;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Kunz H. W., Misra D. N., Hassett A. L. The major histocompatibility complex of the rat. Transplantation. 1987 Jun;43(6):773–785. [PubMed] [Google Scholar]
- Hill M. J. Bile acids and colorectal cancer: hypothesis. Eur J Cancer Prev. 1991 Oct;1 (Suppl 2):69–74. doi: 10.1097/00008469-199110002-00012. [DOI] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall C. J., Ngoi S. S., Goldman I. S., Godwin T., Felix J., DeCosse J. J., Rigas B. Reduced expression of HLA class I and II antigens in colon cancer. Cancer Res. 1990 Dec 15;50(24):8023–8027. [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Nicotra M. R., Frezza F., Pellegrino M. A., Ferrone S. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981 Jan;31(1):75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Prolonged antitumor effect of indomethacin on autochthonous intestinal tumors in rats. J Natl Cancer Inst. 1983 Jun;70(6):1103–1105. [PubMed] [Google Scholar]
- Rosenberg L., Palmer J. R., Zauber A. G., Warshauer M. E., Stolley P. D., Shapiro S. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. J Natl Cancer Inst. 1991 Mar 6;83(5):355–358. doi: 10.1093/jnci/83.5.355. [DOI] [PubMed] [Google Scholar]
- Thun M. J., Namboodiri M. M., Heath C. W., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991 Dec 5;325(23):1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Tsioulias G. J., Triadafilopoulos G., Goldin E., Papavassiliou E. D., Rizos S., Bassioukas P., Rigas B. Expression of HLA class I antigens in sporadic adenomas and histologically normal mucosa of the colon. Cancer Res. 1993 May 15;53(10 Suppl):2374–2378. [PubMed] [Google Scholar]
- Waddell W. R., Ganser G. F., Cerise E. J., Loughry R. W. Sulindac for polyposis of the colon. Am J Surg. 1989 Jan;157(1):175–179. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]