Abstract
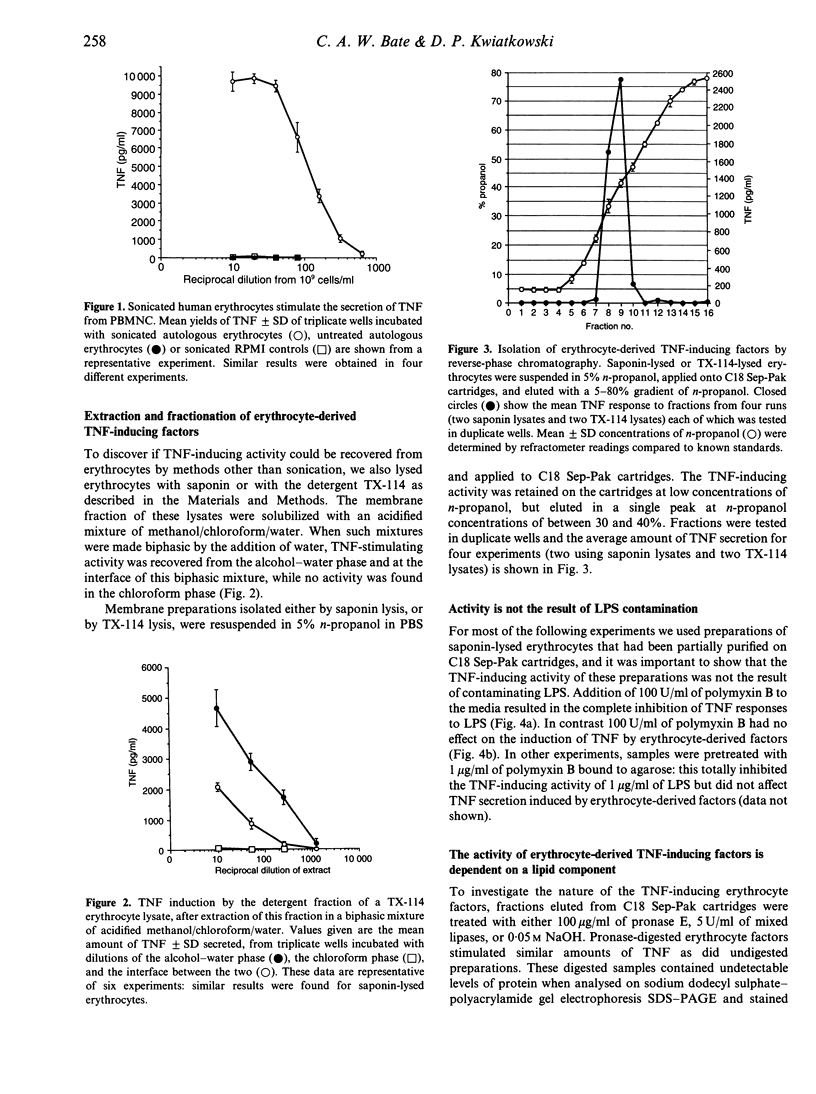
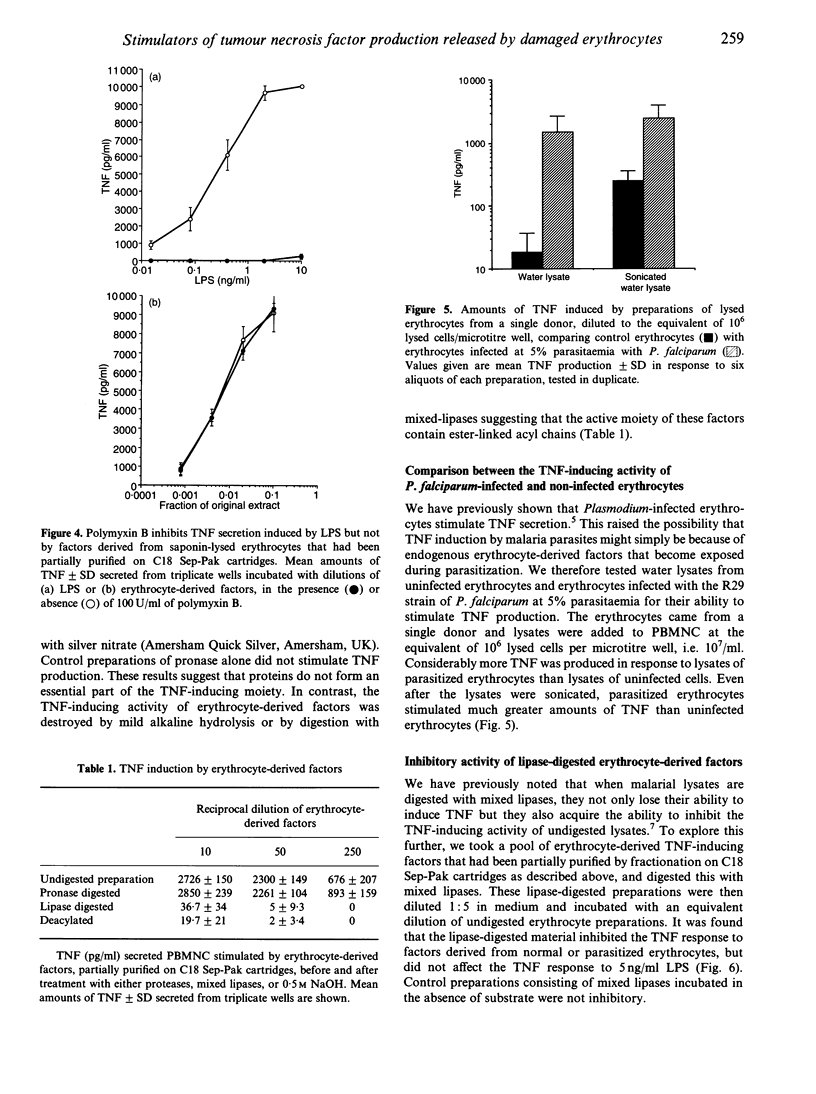
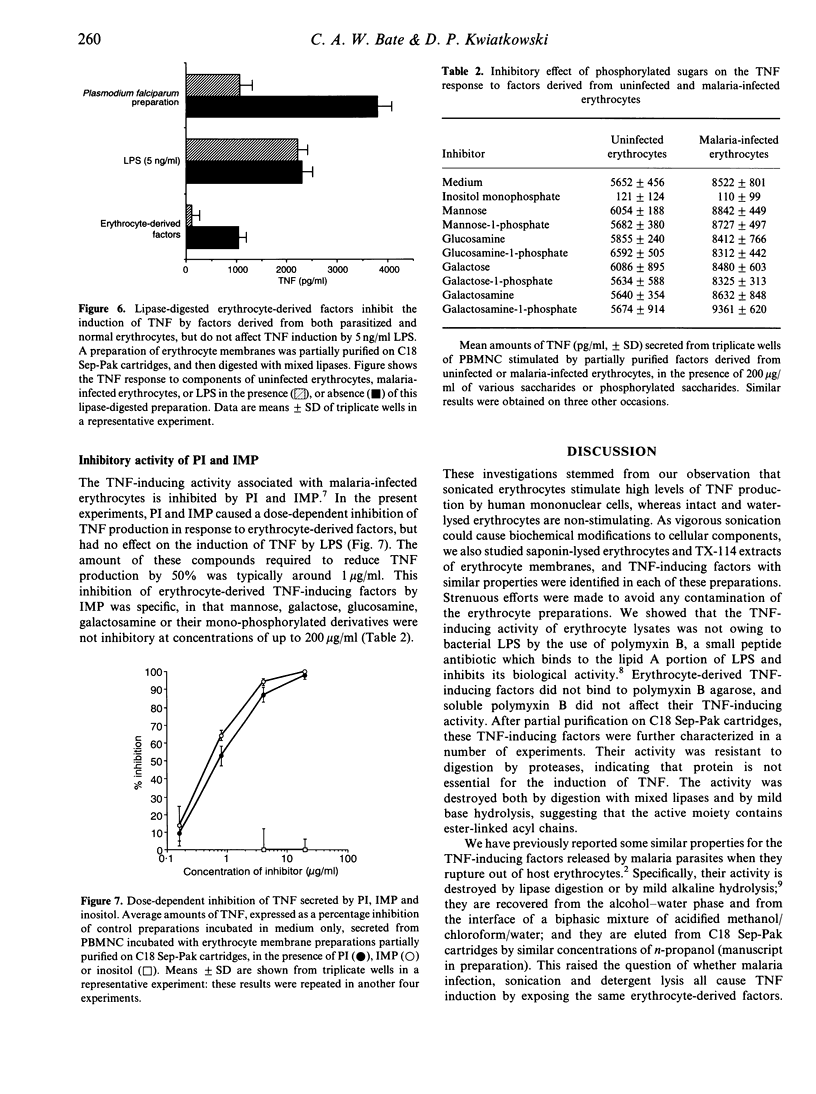
We sought to characterize factors released by sonicated human erythrocytes that stimulate peripheral blood mononuclear cells (PBMC) to release tumour necrosis factor-alpha (TNF). This response is not inhibited by polymyxin B, indicating that contaminating lipopolysaccharide (LPS) is not responsible. When erythrocyte lysates are fractionated by reverse-phase chromatography using a gradient of n-propanol on Sep-Pak C18 cartridges, the TNF-inducing activity elutes as a single peak. The erythrocyte-derived TNF-inducing activity is unaffected by digestion with proteases but is destroyed by mild base hydrolysis or digestion by lipases, indicating that compounds containing ester-linked acyl chains may be essential. These properties are similar to those of TNF stimulators that we have previously identified in erythrocytes infected with malaria parasites, except that the TNF-inducing activity per cell is about 200 times higher in parasitized erythrocytes than in uninfected erythrocytes. Lipase-digested erythrocyte lysates inhibit the TNF-inducing factors of both normal and malaria-infected erythrocytes, suggesting that lipase digestion creates partial structures which compete with active components for macrophage receptors. Such receptors may recognize a common structure that contains an inositol monophosphate (IMP)-like component, as IMP also inhibits the TNF response to erythrocyte-derived factors and to parasite lysates whereas it does not affect the response to LPS. We conclude that lysed erythrocytes release specific cytokine-inducing factors that may contribute to the fever response to non-infectious tissue injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Taverne J., Playfair J. H. Detoxified exoantigens and phosphatidylinositol derivatives inhibit tumor necrosis factor induction by malarial exoantigens. Infect Immun. 1992 May;60(5):1894–1901. doi: 10.1128/iai.60.5.1894-1901.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Başaran Y., Başaran M. M., Babacan K. F., Ener B., Okay T., Gök H., Ozdemir M. Serum tumor necrosis factor levels in acute myocardial infarction and unstable angina pectoris. Angiology. 1993 Apr;44(4):332–337. doi: 10.1177/000331979304400411. [DOI] [PubMed] [Google Scholar]
- Elabbadi N., Ancelin M. L., Vial H. J. Characterization of phosphatidylinositol synthase and evidence of a polyphosphoinositide cycle in Plasmodium-infected erythrocytes. Mol Biochem Parasitol. 1994 Feb;63(2):179–192. doi: 10.1016/0166-6851(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Cannon J. G., Manogue K. R., Cerami A., Dinarello C. A., Greenwood B. M. Tumour necrosis factor production in Falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989 Sep;77(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Machicao F., Mushack J., Seffer E., Ermel B., Häring H. U. Mannose, glucosamine and inositol monophosphate inhibit the effects of insulin on lipogenesis. Further evidence for a role for inositol phosphate-oligosaccharides in insulin action. Biochem J. 1990 Mar 15;266(3):909–916. [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Roberts W. L., Santikarn S., Reinhold V. N., Rosenberry T. L. Structural characterization of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase by fast atom bombardment mass spectrometry. J Biol Chem. 1988 Dec 15;263(35):18776–18784. [PubMed] [Google Scholar]
- Schofield L., Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993 Jan 1;177(1):145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Sarkar D. A., Meager A., Rook G. A., Playfair J. H. Human and murine macrophages produce TNF in response to soluble antigens of Plasmodium falciparum. Parasite Immunol. 1990 Jan;12(1):33–43. doi: 10.1111/j.1365-3024.1990.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]



