Abstract
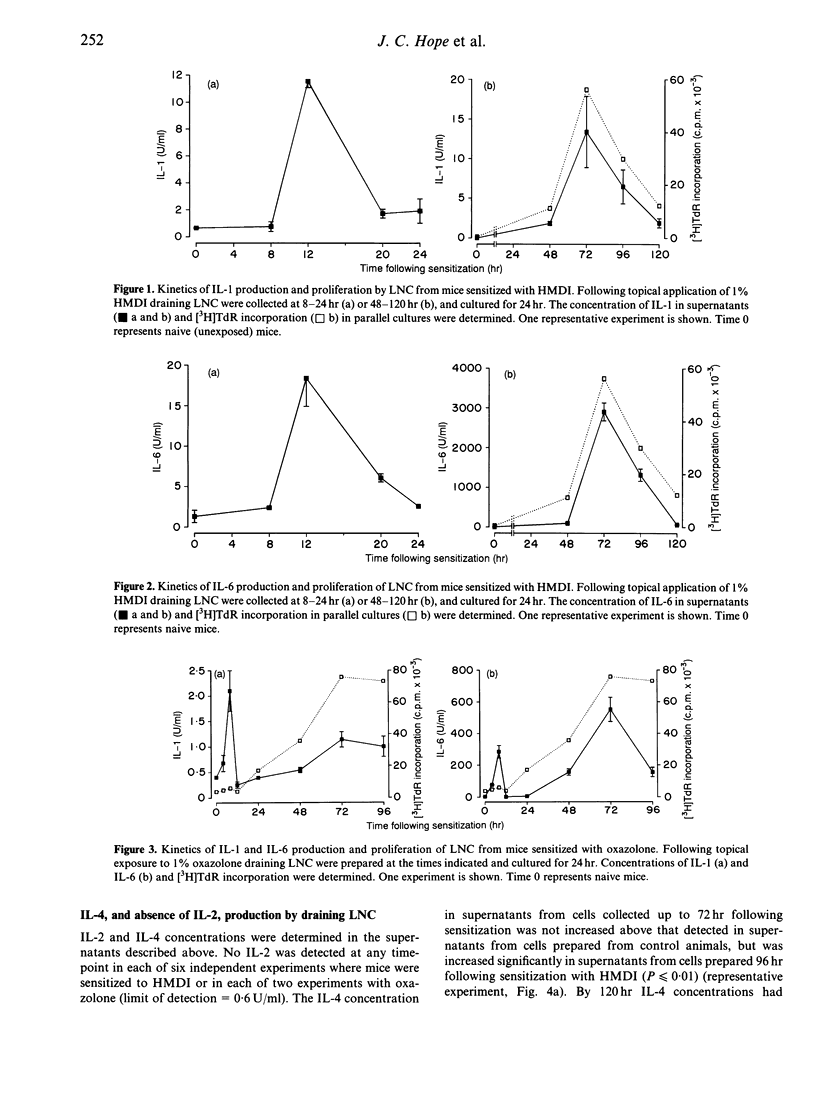
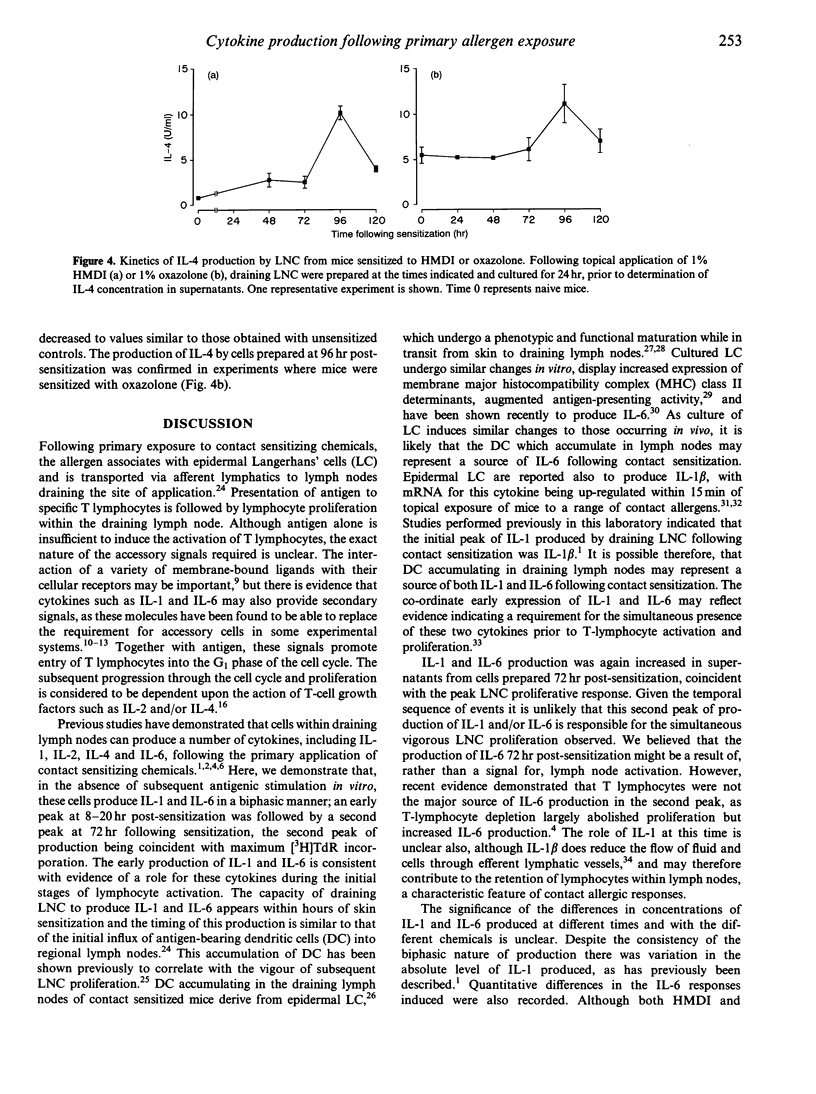
Skin sensitization with chemical allergens is associated with the activation and proliferation of lymphocytes in lymph nodes draining the site of exposure. As lymphocyte activation is regulated by the action of cytokines, we have investigated the nature and kinetics of cytokine production by draining lymph node cells (LNC) from mice, following their primary exposure to chemical allergens. Both interleukin-1 (IL-1) and IL-6 were induced in a biphasic manner following primary exposure of mice to oxazolone or to dicyclohexylmethane-4,4'-diisocyanate (HMDI). The initial phase of production occurred when LNC were prepared from mice 8-20 hr following exposure, while the second peak was coincident with the maximal proliferative response at 72 hr. Increased IL-4 production was observed only when LNC were prepared 96 hr following sensitization. Despite vigorous lymphocyte proliferation there was no evidence for IL-2 production by draining LNC. The ordered and transient pattern of cytokine production that occurs during the afferent phase of contact sensitization suggests that sequential cytokine signals may be involved in regulating the characteristics of the response generated within the draining lymph node.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E., Gillis S., Smith K. A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979 Jan 1;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroja M. L., Ceuppens J. L. More exact quantification of interleukin-2 production by addition of anti-Tac monoclonal antibody to cultures of stimulated lymphocytes. J Immunol Methods. 1987 Apr 16;98(2):267–270. doi: 10.1016/0022-1759(87)90014-7. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Gould S. J., Peters S. W., Kimber I. MHC class II expression by Langerhans' cells and lymph node dendritic cells: possible evidence for maturation of Langerhans' cells following contact sensitization. Immunology. 1991 Nov;74(3):414–419. [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M., Peters S. W., Gould S. J., Kimber I. Intercellular adhesion molecule-1 (ICAM-1) expression by lymph node dendritic cells: comparison with epidermal Langerhans cells. Immunol Lett. 1992 Apr;32(2):105–110. doi: 10.1016/0165-2478(92)90101-s. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Spence L. M., Kimber I. Characterization of murine immune responses to allergenic diisocyanates. Toxicol Appl Pharmacol. 1992 Feb;112(2):190–197. doi: 10.1016/0041-008x(92)90187-w. [DOI] [PubMed] [Google Scholar]
- Enk A. H., Angeloni V. L., Udey M. C., Katz S. I. An essential role for Langerhans cell-derived IL-1 beta in the initiation of primary immune responses in skin. J Immunol. 1993 May 1;150(9):3698–3704. [PubMed] [Google Scholar]
- Enk A. H., Katz S. I. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Gross A., Ben-Sasson S. Z., Paul W. E. Anti-IL-4 diminishes in vivo priming for antigen-specific IL-4 production by T cells. J Immunol. 1993 Mar 15;150(6):2112–2120. [PubMed] [Google Scholar]
- Hanley C. A., Elias R. M., Movat H. Z., Johnston M. G. Suppression of fluid pumping in isolated bovine mesenteric lymphatics by interleukin-1: interaction with prostaglandin E2. Microvasc Res. 1989 Mar;37(2):218–229. doi: 10.1016/0026-2862(89)90039-3. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Holsti M. A., Raulet D. H. IL-6 and IL-1 synergize to stimulate IL-2 production and proliferation of peripheral T cells. J Immunol. 1989 Oct 15;143(8):2514–2519. [PubMed] [Google Scholar]
- Hope J. C., Dearman R. J., Debicki R. J., Kimber I., Hopkins S. J. Interleukin-6 production by draining lymph node cells following primary contact sensitisation of mice: relationship to the proliferative response. Int Arch Allergy Immunol. 1994;103(4):378–383. doi: 10.1159/000236657. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M., Kinnaird A., Jones D. A., Kimber I. Production of interleukin-1 by draining lymph node cells during the induction phase of contact sensitization in mice. Immunology. 1990 Dec;71(4):493–496. [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M. Simple, sensitive and specific bioassay of interleukin-1. J Immunol Methods. 1989 Jun 21;120(2):271–276. doi: 10.1016/0022-1759(89)90252-4. [DOI] [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. Inhibitory influence of IL-4 on human B cell responsiveness. J Immunol. 1988 Jul 1;141(1):164–173. [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- Kinnaird A., Peters S. W., Foster J. R., Kimber I. Dendritic cell accumulation in draining lymph nodes during the induction phase of contact allergy in mice. Int Arch Allergy Appl Immunol. 1989;89(2-3):202–210. doi: 10.1159/000234947. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Munn C. G., Jeevan A., Tang J. M., Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990 Nov 1;145(9):2833–2838. [PubMed] [Google Scholar]
- Lichtman A. H., Kurt-Jones E. A., Abbas A. K. B-cell stimulatory factor 1 and not interleukin 2 is the autocrine growth factor for some helper T lymphocytes. Proc Natl Acad Sci U S A. 1987 Feb;84(3):824–827. doi: 10.1073/pnas.84.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz J., Chain B. Antigen-specific inhibition of IL-2 and IL-3 production in contact sensitivity to TNP. Immunology. 1989 Oct;68(2):185–189. [PMC free article] [PubMed] [Google Scholar]
- Minasi L. E., Kamogawa Y., Carding S., Bottomly K., Flavell R. A. The selective ablation of interleukin 2-producing cells isolated from transgenic mice. J Exp Med. 1993 May 1;177(5):1451–1459. doi: 10.1084/jem.177.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler K. M., Butler L. D. Differential production of IL-2 and IL-4 mRNA in vivo after primary sensitization. J Immunol. 1990 Sep 15;145(6):1734–1739. [PubMed] [Google Scholar]
- Mohler K. M., Butler L. D. Quantitation of cytokine mRNA levels utilizing the reverse transcriptase-polymerase chain reaction following primary antigen-specific sensitization in vivo--I. Verification of linearity, reproducibility and specificity. Mol Immunol. 1991 Apr-May;28(4-5):437–447. doi: 10.1016/0161-5890(91)90157-f. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P. H., Singer A. Similar co-stimulation requirements of CD4+ and CD8+ primary T helper cells: role of IL-1 and IL-6 in inducing IL-2 secretion and subsequent proliferation. Int Immunol. 1992 Mar;4(3):327–335. doi: 10.1093/intimm/4.3.327. [DOI] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Troutt A. B., Kelso A. Contact sensitization to oxazolone: involvement of both interferon-gamma and interleukin-4 in oxazolone-specific Ig and T-cell responses. Immunology. 1993 Feb;78(2):185–192. [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Pike S. E. Interferon-beta 2/interleukin 6 is a co-stimulant for human T lymphocytes. J Immunol. 1988 Sep 1;141(5):1556–1562. [PubMed] [Google Scholar]
- Uyttenhove C., Coulie P. G., Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med. 1988 Apr 1;167(4):1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Wong H. L., Lotze M. T., Wahl L. M., Wahl S. M. Administration of recombinant IL-4 to humans regulates gene expression, phenotype, and function in circulating monocytes. J Immunol. 1992 Apr 1;148(7):2118–2125. [PubMed] [Google Scholar]



