Abstract
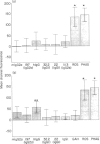
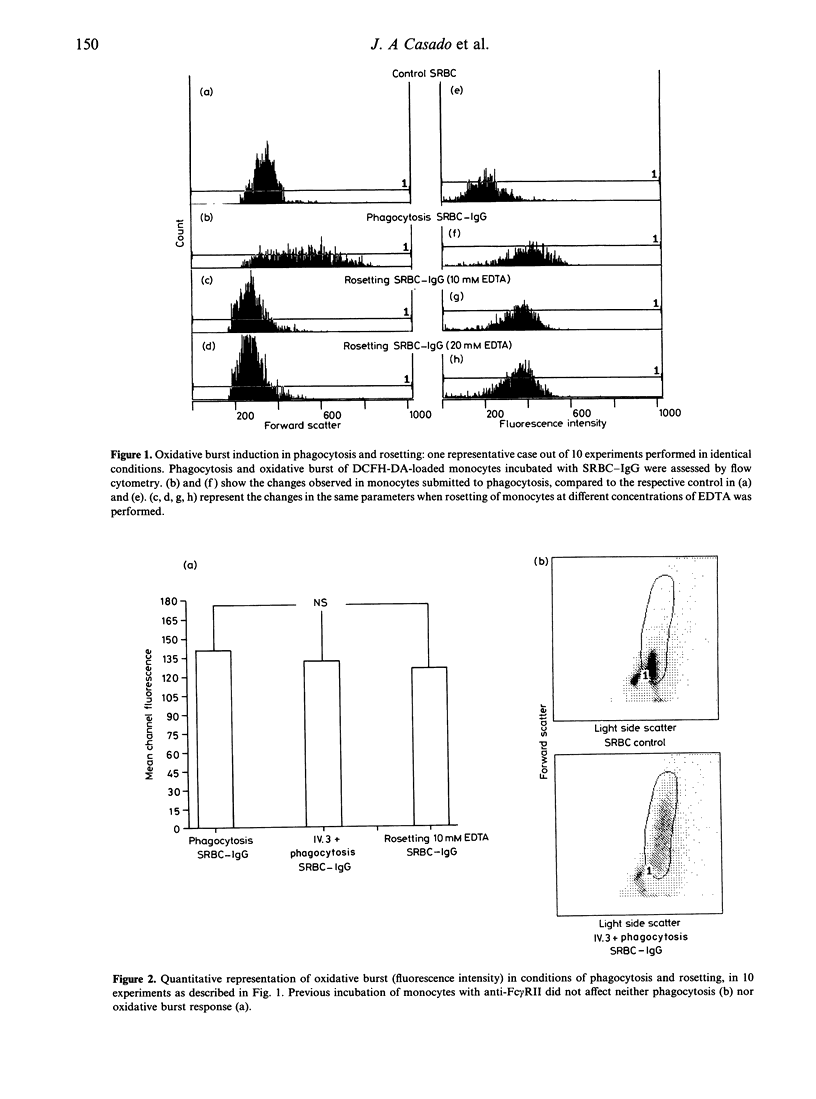
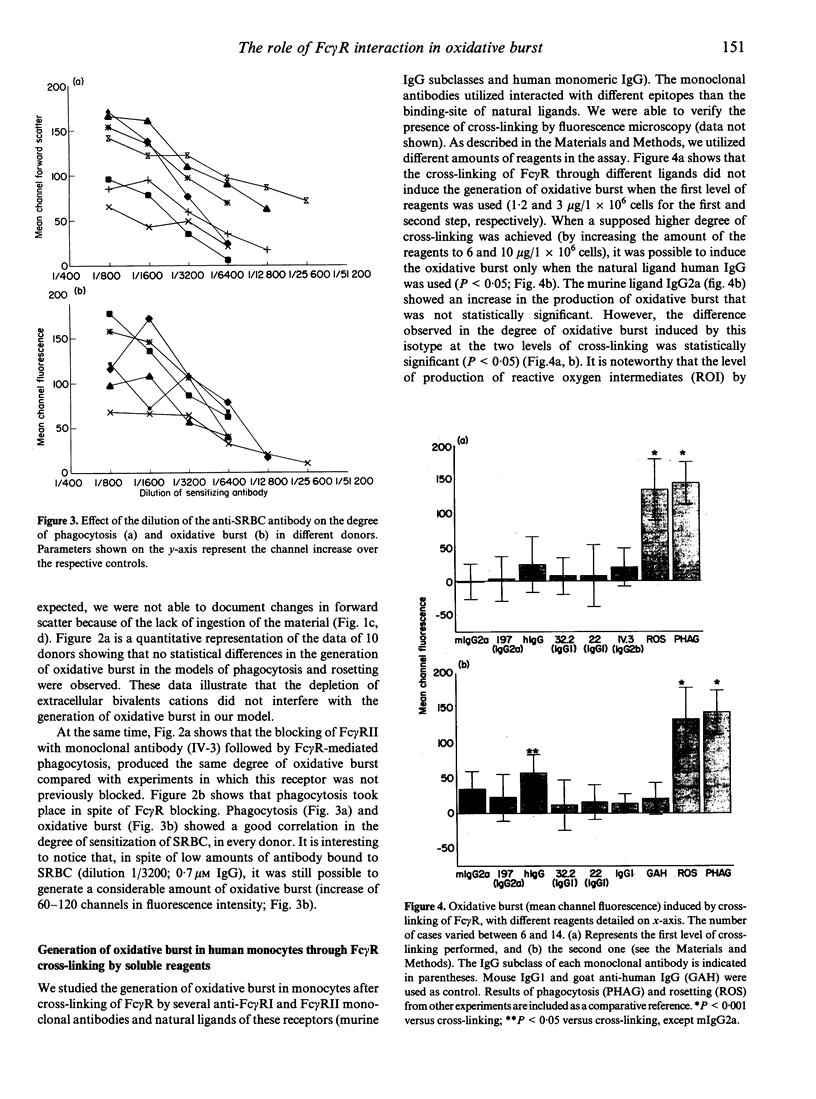
Receptors for the Fc fragment of IgG (Fc gamma R) have a well-documented role in the generation of oxidative burst. It is tempting to speculate that the type of interaction with Fc gamma R could be a mechanism of regulation of this process. Here we report on a comparative study of the induction of oxidative burst in human monocytes activated by means of different types of interaction with Fc gamma R. We studied non-primed monocytes obtained by centrifugal elutriation from healthy donors. These cells were submitted to Fc gamma R interactions following two distinct models: one, using particulate material (IgG-SRBC leading to phagocytosis or rosetting), and another using soluble reagents followed by cross-linking of the receptors (monoclonal antibodies against Fc gamma RI and Fc gamma RII and natural ligands, namely several isotypes of murine and human IgG). Phagocytosis and oxidative burst were studied simultaneously in the monocytes, following the methodology described recently. Human non-primed monocytes were able to generate a very obvious oxidative burst response after activation of Fc gamma R by particulate material. The same response was observed when Fc gamma RII was blocked by monoclonal antibodies. Ingestion was not necessary for activation of the oxidative burst, since the model of rosetting induced a level of burst generation similar to the one obtained in the phagocytic process. Cross-linking of Fc gamma RI by soluble reagents induced production of reactive oxidative intermediates (ROI) only when the ligand-binding site of the receptor was involved. These data lead to the conclusion that Fc gamma R interaction with soluble or particulate material induces oxidative burst in non-primed human monocytes only when the binding site of natural ligands is involved. The type of interaction also determines the efficiency of the generation of ROI. This fact could represent a regulatory mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K. Fc-receptor-mediated phagocytosis: abnormalities associated with diabetes mellitus. Clin Immunol Immunopathol. 1991 Jan;58(1):1–17. doi: 10.1016/0090-1229(91)90144-y. [DOI] [PubMed] [Google Scholar]
- Anderson C. L., Guyre P. M., Whitin J. C., Ryan D. H., Looney R. J., Fanger M. W. Monoclonal antibodies to Fc receptors for IgG on human mononuclear phagocytes. Antibody characterization and induction of superoxide production in a monocyte cell line. J Biol Chem. 1986 Sep 25;261(27):12856–12864. [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Calafat J., Kuijpers T. W., Janssen H., Borregaard N., Verhoeven A. J., Roos D. Evidence for small intracellular vesicles in human blood phagocytes containing cytochrome b558 and the adhesion molecule CD11b/CD18. Blood. 1993 Jun 1;81(11):3122–3129. [PubMed] [Google Scholar]
- Casado J. A., Merino J., Cid J., Subira M. L., Sanchez-Ibarrola A. Simultaneous evaluation of phagocytosis and Fc gamma R-mediated oxidative burst in human monocytes by a simple flow cytometry method. J Immunol Methods. 1993 Feb 26;159(1-2):173–176. doi: 10.1016/0022-1759(93)90155-z. [DOI] [PubMed] [Google Scholar]
- Curnow S. J., Glennie M. J., Stevenson G. T. Cytoplasmic calcium fluxes induced in cytotoxic effector cells by engagement of Fc gamma receptors I, II, and III. Scand J Immunol. 1992 Aug;36(2):221–231. doi: 10.1111/j.1365-3083.1992.tb03094.x. [DOI] [PubMed] [Google Scholar]
- Debets J. M., Van de Winkel J. G., Ceuppens J. L., Dieteren I. E., Buurman W. A. Cross-linking of both Fc gamma RI and Fc gamma RII induces secretion of tumor necrosis factor by human monocytes, requiring high affinity Fc-Fc gamma R interactions. Functional activation of Fc gamma RII by treatment with proteases or neuraminidase. J Immunol. 1990 Feb 15;144(4):1304–1310. [PubMed] [Google Scholar]
- Guyre P. M., Graziano R. F., Vance B. A., Morganelli P. M., Fanger M. W. Monoclonal antibodies that bind to distinct epitopes on Fc gamma RI are able to trigger receptor function. J Immunol. 1989 Sep 1;143(5):1650–1655. [PubMed] [Google Scholar]
- Hassan N. F., Campbell D. E., Douglas S. D. Phorbol myristate acetate induced oxidation of 2',7'-dichlorofluorescin by neutrophils from patients with chronic granulomatous disease. J Leukoc Biol. 1988 Apr;43(4):317–322. doi: 10.1002/jlb.43.4.317. [DOI] [PubMed] [Google Scholar]
- Hoffman T., Tripathi A. K., Lee Y. L., Bonvini E., Golding B. Inflammatory mediator release from human monocytes via immobilized Fc receptors. Its potential role in adverse reactions to systemic monoclonal antibody therapy. Transplantation. 1992 Aug;54(2):343–346. doi: 10.1097/00007890-199208000-00027. [DOI] [PubMed] [Google Scholar]
- Huizinga T. W., van Kemenade F., Koenderman L., Dolman K. M., von dem Borne A. E., Tetteroo P. A., Roos D. The 40-kDa Fc gamma receptor (FcRII) on human neutrophils is essential for the IgG-induced respiratory burst and IgG-induced phagocytosis. J Immunol. 1989 Apr 1;142(7):2365–2369. [PubMed] [Google Scholar]
- Kaffenberger W., Clasen B. P., van Beuningen D. The respiratory burst of neutrophils, a prognostic parameter in head and neck cancer? Clin Immunol Immunopathol. 1992 Jul;64(1):57–62. doi: 10.1016/0090-1229(92)90059-w. [DOI] [PubMed] [Google Scholar]
- Kimberly R. P., Meryhew N. L., Runquist O. A. Mononuclear phagocyte function in SLE. I. Bipartite Fc- and complement-dependent dysfunction. J Immunol. 1986 Jul 1;137(1):91–96. [PubMed] [Google Scholar]
- Klaassen R. J., Ouwehand W. H., Huizinga T. W., Engelfriet C. P., von dem Borne A. E. The Fc-receptor III of cultured human monocytes. Structural similarity with FcRIII of natural killer cells and role in the extracellular lysis of sensitized erythrocytes. J Immunol. 1990 Jan 15;144(2):599–606. [PubMed] [Google Scholar]
- Klygis L. M., Reisberg B. E. Spinal epidural abscess caused by group G streptococci. Am J Med. 1991 Jul;91(1):89–90. doi: 10.1016/0002-9343(91)90078-c. [DOI] [PubMed] [Google Scholar]
- Kávai M., Gyimesi E., Szücs G., Szegedi G. Binding and endocytosis of erythrocytes sensitized with rabbit IgG via Fc gamma receptors of human monocytes. Immunology. 1991 Dec;74(4):657–660. [PMC free article] [PubMed] [Google Scholar]
- Looney R. J., Abraham G. N., Anderson C. L. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986 Mar 1;136(5):1641–1647. [PubMed] [Google Scholar]
- MacIntyre E. A., Roberts P. J., Abdul-Gaffar R., O'Flynn K., Pilkington G. R., Farace F., Morgan J., Linch D. C. Mechanism of human monocyte activation via the 40-kDa Fc receptor for IgG. J Immunol. 1988 Dec 15;141(12):4333–4343. [PubMed] [Google Scholar]
- MacIntyre E. A., Roberts P. J., Jones M., Van der Schoot C. E., Favalaro E. J., Tidman N., Linch D. C. Activation of human monocytes occurs on cross-linking monocytic antigens to an Fc receptor. J Immunol. 1989 Apr 1;142(7):2377–2383. [PubMed] [Google Scholar]
- Macintyre E. A., Roberts P. J., Abdul-Gaffar R., Morgan J., Linch D. C. Activation of monocytic cells by monoclonal antibodies to the CD11a/18 (LFA-1) complex: mediation by Fc receptor. Immunology. 1990 Apr;69(4):574–579. [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. Oxygen free radicals linked to many diseases. Science. 1987 Jan 30;235(4788):529–531. doi: 10.1126/science.3810154. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Werlen G., Clerget M., Pittet D., Rossier M. F., Capponi A. M. 1,25-dihydroxyvitamin D3 induces responsiveness to the chemotactic peptide f-Met-Leu-Phe in the human monocytic line U937: dissociation between calcium and oxidative metabolic responses. J Leukoc Biol. 1989 May;45(5):381–388. doi: 10.1002/jlb.45.5.381. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Devereux S., Pilkington G. R., Linch D. C. Fc gamma RII-mediated superoxide production by phagocytes is augmented by GM-CSF without a change in Fc gamma RII expression. J Leukoc Biol. 1990 Sep;48(3):247–257. doi: 10.1002/jlb.48.3.247. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Salmon J. E., Kimberly R. P., Gibofsky A., Fotino M. Defective mononuclear phagocyte function in systemic lupus erythematosus: dissociation of Fc receptor-ligand binding and internalization. J Immunol. 1984 Nov;133(5):2525–2531. [PubMed] [Google Scholar]
- Scholl P. R., Ahern D., Geha R. S. Protein tyrosine phosphorylation induced via the IgG receptors Fc gamma Ri and Fc gamma RII in the human monocytic cell line THP-1. J Immunol. 1992 Sep 1;149(5):1751–1757. [PubMed] [Google Scholar]
- Scully S. P., Segel G. B., Lichtman M. A. Relationship of superoxide production to cytoplasmic free calcium in human monocytes. J Clin Invest. 1986 Apr;77(4):1349–1356. doi: 10.1172/JCI112440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger H., Gadd S. J., Eher R., Majdic O., Schreiber W., Kasinrerk W., Strass B., Schnabl E., Knapp W. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol. 1990 Dec 1;145(11):3889–3897. [PubMed] [Google Scholar]
- Vlassara H., Moldawer L., Chan B. Macrophage/monocyte receptor for nonenzymatically glycosylated protein is upregulated by cachectin/tumor necrosis factor. J Clin Invest. 1989 Dec;84(6):1813–1820. doi: 10.1172/JCI114366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller J. M., Rothberg L., Landay A. L. Evaluation of human monocyte oxidative metabolism utilizing a flow cytometric assay. Clin Exp Immunol. 1989 Oct;78(1):91–96. [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Nibbering P. H., van Furth R. Cytosolic free calcium is essential for immunoglobulin G-stimulated intracellular killing of Staphylococcus aureus by human monocytes. Infect Immun. 1992 Aug;60(8):3092–3097. doi: 10.1128/iai.60.8.3092-3097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Winkel J. G., Capel P. J. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993 May;14(5):215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]