Abstract
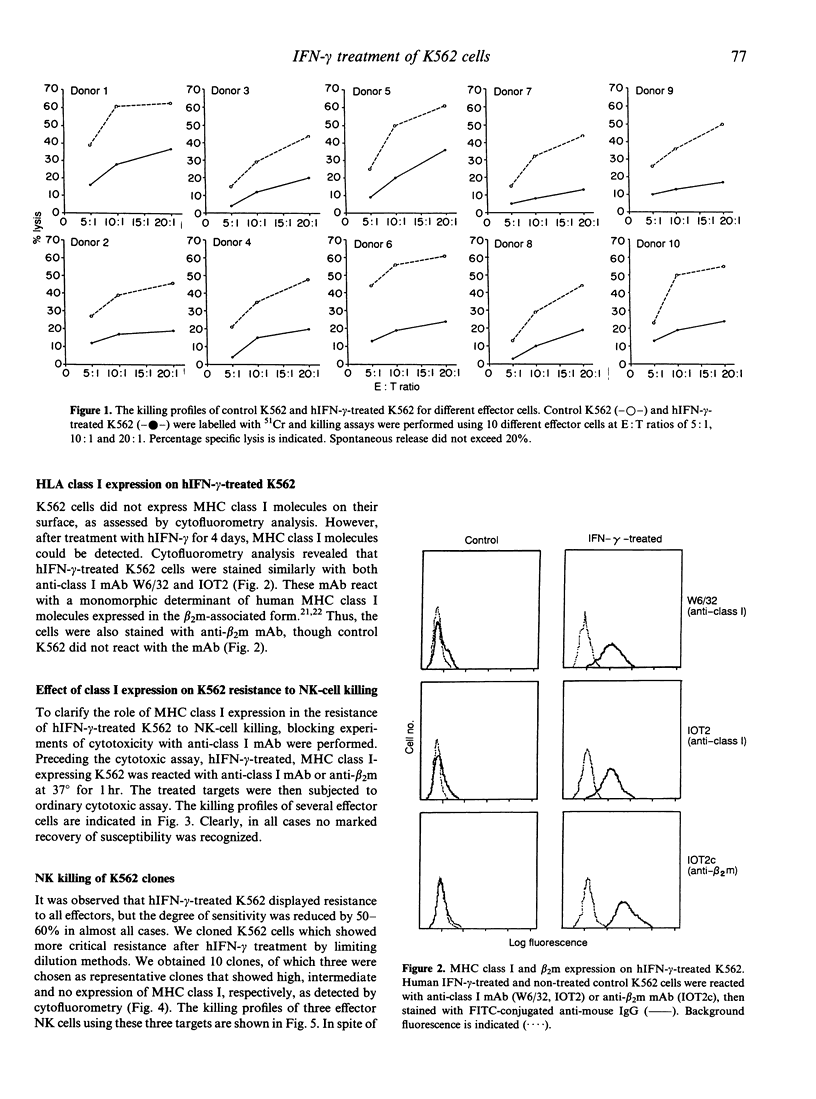
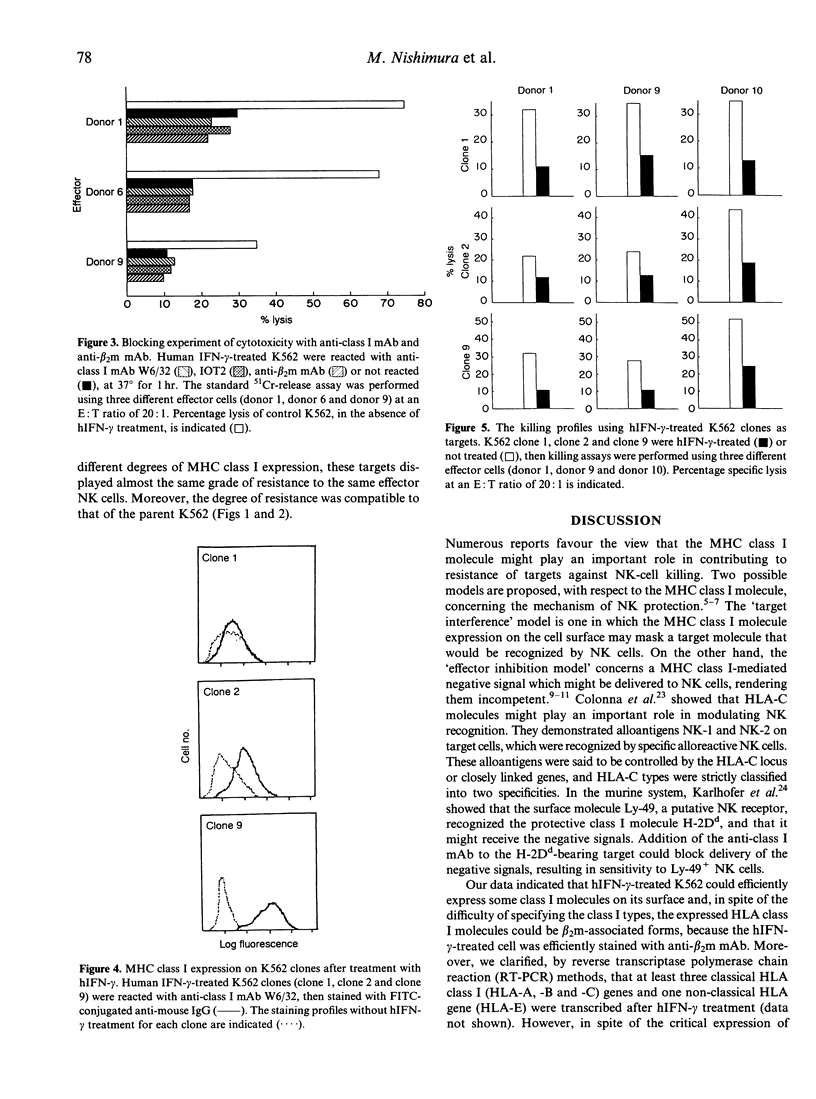
Prior study has revealed that the expression of certain major histocompatibility complex (MHC) class I molecules on the membranes of normal or transformed cells can prevent natural killer (NK)-cell-mediated killing. This has been explained by either (1) the target interference model, or (2) the effector inhibition model. In both cases, MHC class I molecules are concerned but the precise mechanisms are still unclear. The erythroleukaemia K562 cell is known as a NK-sensitive target. This sensitivity has been explained mostly as being due to lack of MHC class I antigens on the K562 membrane. However, several recent studies have indicated that the expression of MHC class I antigens on the cell does not solely explain the protection against NK cells. To elucidate the mechanism of NK-cell-mediated killing, we investigated the killing profiles of the K562 cells by NK cells. Previous studies indicated that the NK-sensitive K562 cells can express some MHC class I antigens on their surface, and become protective against NK-cell killing after treatment with human interferon-gamma (hIFN-gamma). In the present study, we show that this resistance is not rendered by MHC class I expression, because addition of anti-MHC class I monoclonal antibodies (mAb) in the killing assay of hIFN-gamma-treated K562 cells, which express MHC class I antigens as the target, did not restore the sensitivity to NK cells. Moreover, we show that spontaneously occurring K562 clones which could not express MHC class I antigens even after hIFN-gamma treatment, could be protected after treatment with the cytokine. Taken together, these results strongly suggest that the susceptibility of K562 cells to NK cell killing is due to some elements distinct from those related to MHC class I antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colonna M., Brooks E. G., Falco M., Ferrara G. B., Strominger J. L. Generation of allospecific natural killer cells by stimulation across a polymorphism of HLA-C. Science. 1993 May 21;260(5111):1121–1124. doi: 10.1126/science.8493555. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984 Sep;38(3):287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- Giorda R., Rudert W. A., Vavassori C., Chambers W. H., Hiserodt J. C., Trucco M. NKR-P1, a signal transduction molecule on natural killer cells. Science. 1990 Sep 14;249(4974):1298–1300. doi: 10.1126/science.2399464. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Holscher M., Givan A. L., Brooks C. G. The effect of transfected MHC class I genes on sensitivity to natural killer cells. Immunology. 1991 May;73(1):44–51. [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Yabe T., McSherry C., Bach F. H. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991 Apr 1;173(4):1017–1020. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. S., Schoon R. A., Leibson P. J. MHC class I expression on tumor targets inhibits natural killer cell-mediated cytotoxicity without interfering with target recognition. J Immunol. 1993 Feb 15;150(4):1429–1436. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H., Hackett J., Jr, Tutt M., Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986 Nov 1;137(9):2735–2739. [PubMed] [Google Scholar]
- Leiden J. M., Karpinski B. A., Gottschalk L., Kornbluth J. Susceptibility to natural killer cell-mediated cytolysis is independent of the level of target cell class I HLA expression. J Immunol. 1989 Mar 15;142(6):2140–2147. [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Litwin V., Gumperz J., Parham P., Phillips J. H., Lanier L. L. Specificity of HLA class I antigen recognition by human NK clones: evidence for clonal heterogeneity, protection by self and non-self alleles, and influence of the target cell type. J Exp Med. 1993 Oct 1;178(4):1321–1336. doi: 10.1084/jem.178.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Malnati M. S., Lusso P., Ciccone E., Moretta A., Moretta L., Long E. O. Recognition of virus-infected cells by natural killer cell clones is controlled by polymorphic target cell elements. J Exp Med. 1993 Sep 1;178(3):961–969. doi: 10.1084/jem.178.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunaga S., Kuwata S., Tokunaga K., Uchikawa C., Takahashi K., Akaza T., Mitomi Y., Juji T. Family study on HLA-DPB1 polymorphism: linkage analysis with HLA-DR/DQ and two "new" alleles. Hum Immunol. 1992 Jul;34(3):203–211. doi: 10.1016/0198-8859(92)90113-2. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Itoh T. Higher level expression of lymphocyte function-associated antigen-1 (LFA-1) on in vivo natural killer cells. Eur J Immunol. 1988 Dec;18(12):2077–2080. doi: 10.1002/eji.1830181231. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Immunology. A sense of something missing. Nature. 1992 Jul 2;358(6381):21–22. doi: 10.1038/358021a0. [DOI] [PubMed] [Google Scholar]
- Rebaï N., Malissen B. Structural and genetic analyses of HLA class I molecules using monoclonal xenoantibodies. Tissue Antigens. 1983 Aug;22(2):107–117. doi: 10.1111/j.1399-0039.1983.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Reiter Z., Reiter Y., Fishelson Z., Shinitzky M., Kessler A., Loyter A., Nussbaum O., Rubinstein M. Resistance to NK cell-mediated cytotoxicity (in K-562 cells) does not correlate with class I MHC antigen levels. Immunobiology. 1991 Sep;183(1-2):23–39. doi: 10.1016/S0171-2985(11)80183-X. [DOI] [PubMed] [Google Scholar]
- Routes J. M. IFN increases class I MHC antigen expression on adenovirus-infected human cells without inducing resistance to natural killer cell killing. J Immunol. 1992 Oct 1;149(7):2372–2377. [PubMed] [Google Scholar]
- Shimizu Y., DeMars R. Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur J Immunol. 1989 Mar;19(3):447–451. doi: 10.1002/eji.1830190306. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Alexander J., Payne J. A., Dawson J. R., Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkus W. J., Dawson J. R. Target structures involved in natural killing (NK): characteristics, distribution, and candidate molecules. Crit Rev Immunol. 1991;10(5):393–416. [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara H., Bloom E. T. The IL-2 receptor beta subunit is absolutely required for mediating the IL-2-induced activation of NK activity and proliferative activity of human large granular lymphocytes. Immunology. 1990 May;70(1):111–115. [PMC free article] [PubMed] [Google Scholar]
- Yabe T., McSherry C., Bach F. H., Fisch P., Schall R. P., Sondel P. M., Houchins J. P. A multigene family on human chromosome 12 encodes natural killer-cell lectins. Immunogenetics. 1993;37(6):455–460. doi: 10.1007/BF00222470. [DOI] [PubMed] [Google Scholar]
- Yokoyama W. M., Kehn P. J., Cohen D. I., Shevach E. M. Chromosomal location of the Ly-49 (A1, YE1/48) multigene family. Genetic association with the NK 1.1 antigen. J Immunol. 1990 Oct 1;145(7):2353–2358. [PubMed] [Google Scholar]


