Abstract
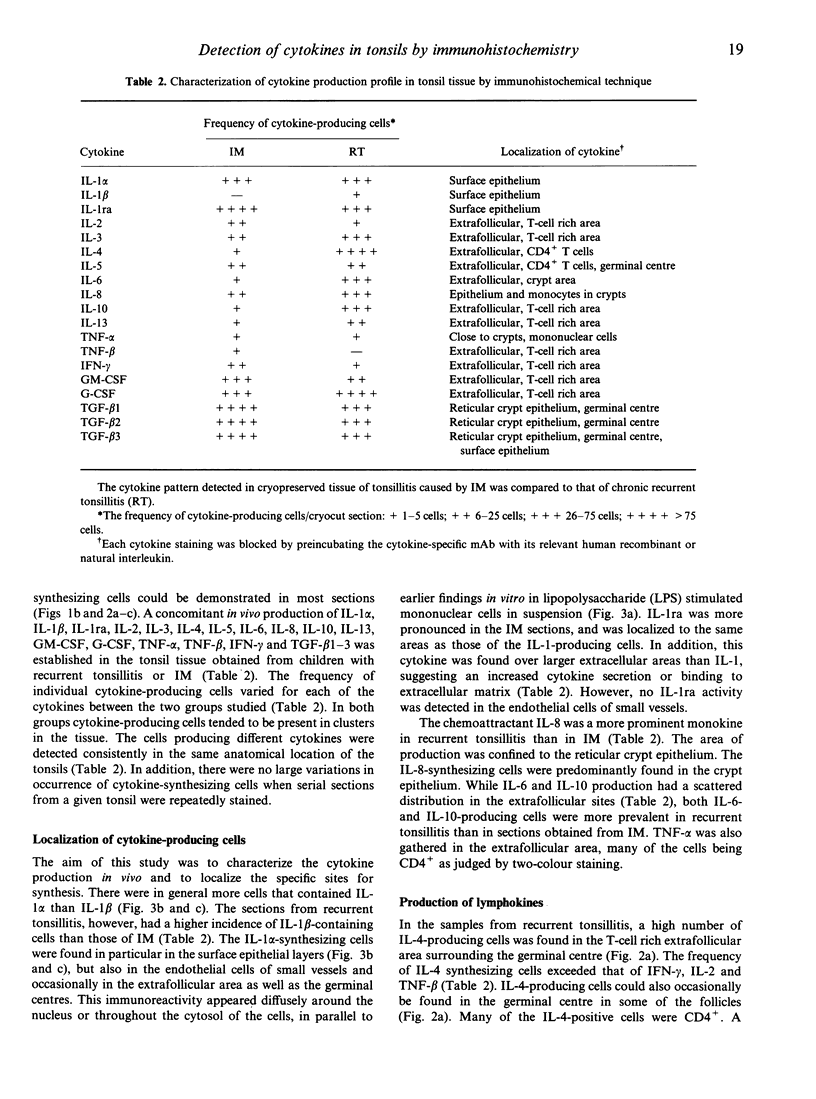
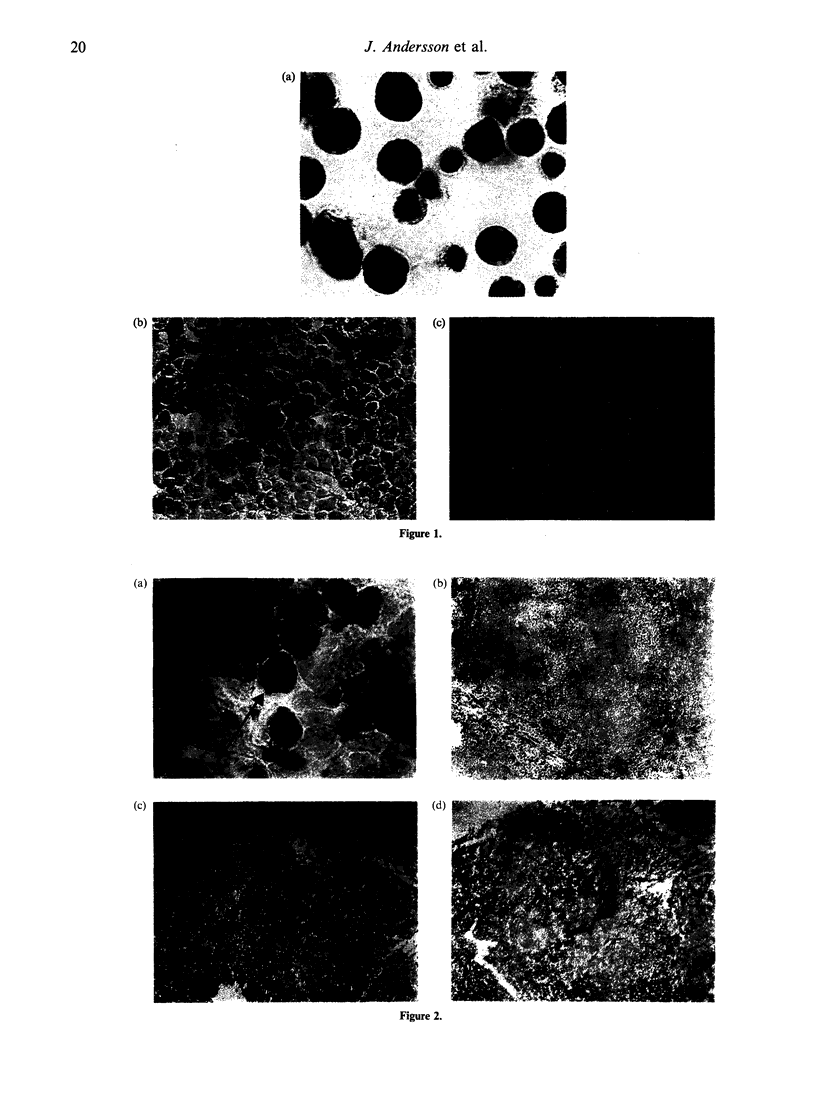
Accumulating data indicate that cytokines, peptides involved in regulation of both physiological and pathological immune responses, are produced predominantly at the site of local antigen stimulation. Cytokine-producing cells were detected at the protein level in human tonsil tissue obtained from children with recurrent tonsillitis or infectious mononucleosis (IM). Concomitant production of 19 different human cytokines, interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-1 receptor antagonist (ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, tumour necrosis factor-alpha (TNF-alpha), TNF-beta, interferon-gamma (IFN-gamma) and transforming growth factor-beta 1-3 (TGF-beta 1-3), was identified at a single-cell level by indirect immunohistochemical staining procedures and use of carefully selected cytokine-specific antibodies (Ab). Fresh frozen sections were fixed with 4% paraformaldehyde and permeabilized by 0.1% saponin treatment, eluting cholesterol from the cell-surface membrane and the Golgi complex. The intracellular localization of all cytokines, except IL-1 and IL-1ra, was demonstrated by a characteristic local cytoplasmic perinuclear configuration in producer cells. In addition, the immunoreactivity for certain cytokines (IL-2, IL-4, IL-5, G-CSF and GM-CSF) was expressed on the cell membranes and extended over a large extracellular area encompassing the producer cell. Localization of the cytokine to the Golgi organelle was established by co-staining with a monoclonal antibody (mAb) specific to the Golgi complex. Both the extra- and intracellular cytokine staining reactions could be blocked by preincubation of the cytokine-specific Ab with the corresponding purified natural or recombinant cytokine. A complex cytokine pattern was established in both groups studied, where most T-helper type 1 (Th1) and Th2 lymphokines were expressed in the tonsils but at different frequencies and localizations. Cells expressing IL-4, IL-5, IL-10 and IL-13, (Th2 response) were evident at higher frequencies in recurrent tonsillitis compared to sections from IM, which were associated with a more pronounced IL-2, IFN-gamma and TNF-beta expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Roncarolo M. G., Yssel H., Andersson U., Gleich G. J., Silver J. E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992 Jun;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Agace W., Hedges S., Andersson U., Andersson J., Ceska M., Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993 Feb;61(2):602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Andersson J., Andersson U. Characterization of cytokine production in infectious mononucleosis studied at a single-cell level in tonsil and peripheral blood. Clin Exp Immunol. 1993 Apr;92(1):7–13. doi: 10.1111/j.1365-2249.1993.tb05939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Björk L., Dinarello C. A., Towbin H., Andersson U. Lipopolysaccharide induces human interleukin-1 receptor antagonist and interleukin-1 production in the same cell. Eur J Immunol. 1992 Oct;22(10):2617–2623. doi: 10.1002/eji.1830221022. [DOI] [PubMed] [Google Scholar]
- Andersson U., Adolf G., Dohlsten M., Möller G., Sjögren H. O. Characterization of individual tumor necrosis factor alpha-and beta-producing cells after polyclonal T cell activation. J Immunol Methods. 1989 Oct 24;123(2):233–240. doi: 10.1016/0022-1759(89)90227-5. [DOI] [PubMed] [Google Scholar]
- Bogen S. A., Fogelman I., Abbas A. K. Analysis of IL-2, IL-4, and IFN-gamma-producing cells in situ during immune responses to protein antigens. J Immunol. 1993 May 15;150(10):4197–4205. [PubMed] [Google Scholar]
- Bradding P., Feather I. H., Wilson S., Bardin P. G., Heusser C. H., Holgate S. T., Howarth P. H. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993 Oct 1;151(7):3853–3865. [PubMed] [Google Scholar]
- Chizzonite R., Truitt T., Podlaski F. J., Wolitzky A. G., Quinn P. M., Nunes P., Stern A. S., Gately M. K. IL-12: monoclonal antibodies specific for the 40-kDa subunit block receptor binding and biologic activity on activated human lymphoblasts. J Immunol. 1991 Sep 1;147(5):1548–1556. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Dolhain R. J., Andersson U., ter Haar N. T., Brinkman B. M., Verweij C. L., Daha M. R., Breedveld F. C., Miltenburg A. M. Detection of intracellular interferon-gamma by light microscopy using an immunoperoxidase technique: correlation with the corresponding mRNA and protein product. J Leukoc Biol. 1993 Dec;54(6):545–551. doi: 10.1002/jlb.54.6.545. [DOI] [PubMed] [Google Scholar]
- Holder M. J., Knox K., Gordon J. Factors modifying survival pathways of germinal center B cells. Glucocorticoids and transforming growth factor-beta, but not cyclosporin A or anti-CD19, block surface immunoglobulin-mediated rescue from apoptosis. Eur J Immunol. 1992 Oct;22(10):2725–2728. doi: 10.1002/eji.1830221037. [DOI] [PubMed] [Google Scholar]
- Jung T., Schauer U., Heusser C., Neumann C., Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993 Feb 26;159(1-2):197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- Kuper C. F., Koornstra P. J., Hameleers D. M., Biewenga J., Spit B. J., Duijvestijn A. M., van Breda Vriesman P. J., Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992 Jun;13(6):219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- McKenzie A. N., Culpepper J. A., de Waal Malefyt R., Brière F., Punnonen J., Aversa G., Sato A., Dang W., Cocks B. G., Menon S. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Chalon P., Derocq J. M., Dumont X., Guillemot J. C., Kaghad M., Labit C., Leplatois P., Liauzun P., Miloux B. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993 Mar 18;362(6417):248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Munoz C., Misset B., Fitting C., Blériot J. P., Carlet J., Cavaillon J. M. Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur J Immunol. 1991 Sep;21(9):2177–2184. doi: 10.1002/eji.1830210928. [DOI] [PubMed] [Google Scholar]
- Nadal D., Albini B., Chen C. Y., Schläpfer E., Bernstein J. M., Ogra P. L. Distribution and engraftment patterns of human tonsillar mononuclear cells and immunoglobulin-secreting cells in mice with severe combined immunodeficiency: role of the Epstein-Barr virus. Int Arch Allergy Appl Immunol. 1991;95(4):341–351. doi: 10.1159/000235471. [DOI] [PubMed] [Google Scholar]
- Nadal D., Albini B., Schläpfer E., Chen C., Brodsky L., Ogra P. L. Tissue distribution of mucosal antibody-producing cells specific for respiratory syncytial virus in severe combined immune deficiency (SCID) mice engrafted with human tonsils. Clin Exp Immunol. 1991 Sep;85(3):358–364. doi: 10.1111/j.1365-2249.1991.tb05732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson A., Miyazono K., Kanzaki T., Colosetti P., Engström U., Heldin C. H. Transforming growth factor-beta 1, -beta 2, and -beta 3 secreted by a human glioblastoma cell line. Identification of small and different forms of large latent complexes. J Biol Chem. 1992 Sep 25;267(27):19482–19488. [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Quiding M., Granström G., Nordström I., Ferrua B., Holmgren J., Czerkinsky C. High frequency of spontaneous interferon-gamma-producing cells in human tonsils: role of local accessory cells and soluble factors. Clin Exp Immunol. 1993 Jan;91(1):157–163. doi: 10.1111/j.1365-2249.1993.tb03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiding M., Nordström I., Kilander A., Andersson G., Hanson L. A., Holmgren J., Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest. 1991 Jul;88(1):143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Sander B., Andersson J., Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991 Feb;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Hall G. L., Limjuco G., Chin J., Schmidt J. A. Interleukin 1 beta is localized in the cytoplasmic ground substance but is largely absent from the Golgi apparatus and plasma membranes of stimulated human monocytes. J Exp Med. 1988 Feb 1;167(2):389–407. doi: 10.1084/jem.167.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Waegell W., Beernink H., Dasch J. R. Transforming growth factor-beta 1 is required for secretion of IgG of all subclasses by LPS-activated murine B cells in vitro. J Immunol. 1993 Nov 1;151(9):4625–4636. [PubMed] [Google Scholar]
- Sugiyama M., Uekawa M., Yamane H., Takeda M., Sakamoto H., Nishimoto A., Nakai Y. Influence of IL-6 on proliferation and differentiation of tonsillar lymphocytes and detection of IL-6 producing cells in tonsil. Acta Otolaryngol Suppl. 1991;486:245–253. doi: 10.3109/00016489109135002. [DOI] [PubMed] [Google Scholar]
- Symons J. A., Eastgate J. A., Duff G. W. Purification and characterization of a novel soluble receptor for interleukin 1. J Exp Med. 1991 Nov 1;174(5):1251–1254. doi: 10.1084/jem.174.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zee K. J., Kohno T., Fischer E., Rock C. S., Moldawer L. L., Lowry S. F. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vlasselaer P., Punnonen J., de Vries J. E. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992 Apr 1;148(7):2062–2067. [PubMed] [Google Scholar]






