Abstract
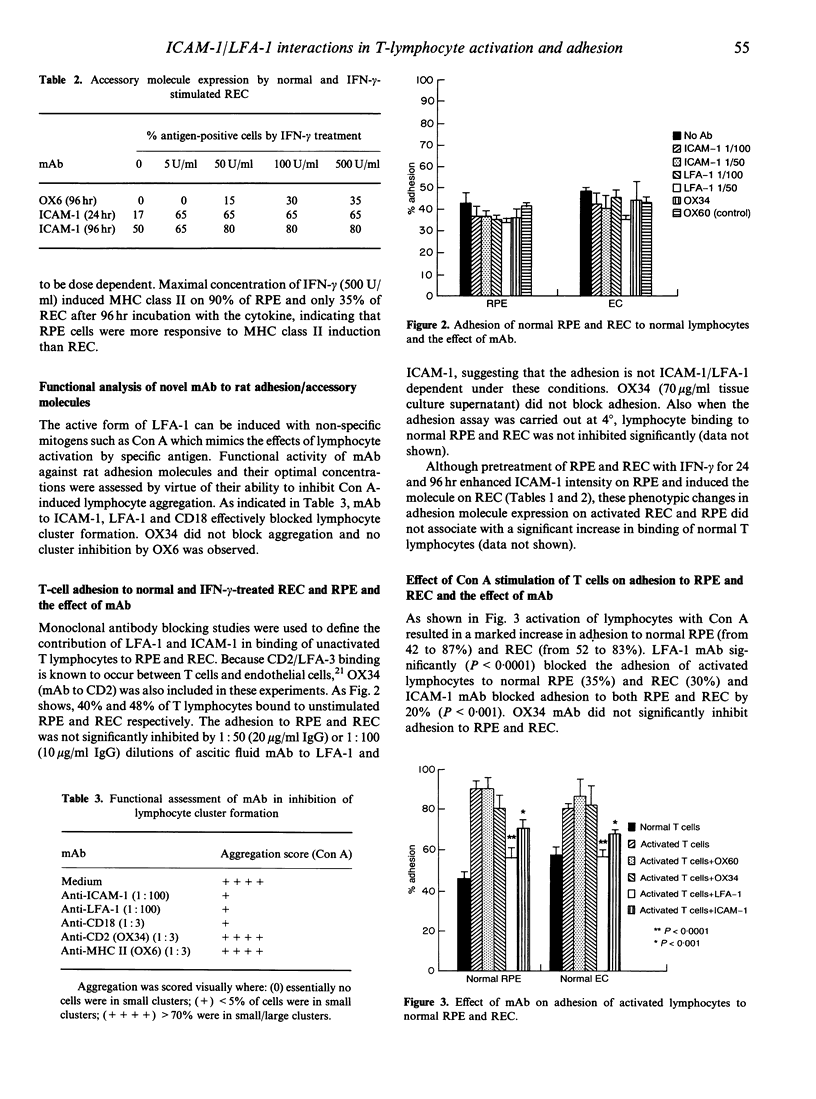
To identify the signals involved in the adhesion and subsequent migration of lymphocytes across the endothelium (REC) and pigment epithelium (RPE) of the blood-retina barrier we have studied the effects of monoclonal antibodies (mAb) to rat adhesion/accessory molecules on the binding of normal and concanavalin A (Con A)-activated rat spleen lymphocytes to cultured unstimulated and interferon-gamma (IFN-gamma)-stimulated RPE and REC. Forty to 48% of unactivated T cells were found to bind to normal REC or RPE by leucocyte function-associated antigen-1/intercellular adhesion molecule-1 (LFA-1/ICAM-1)-independent mechanisms, despite constitutive expression of ICAM-1 by the RPE cells and LFA-1 by the T cells. Con A-activated lymphocytes showed an enhanced adhesion to both RPE and REC. However, IFN-gamma-stimulated RPE and REC did not demonstrate a significant increase in adhesiveness for normal lymphocytes highlighting the importance of lymphocyte integrin activation from low-affinity to high-affinity state. Activated lymphocyte adhesion to unstimulated RPE and REC was significantly blocked by LFA-1 mAb (35%, P < 0.0001) and ICAM-1 mAb (20%, P < 0.001). Inhibition of adhesion by antibody to CD2 was not significant. Both ICAM-1 and LFA-1 mAb also significantly (P < 0.05) blocked antigen presentation following retinal extract stimulation of lymphocytes from immunized rats in proliferation assay. These data suggest that the ICAM-1/LFA-1 system is important in lymphocyte trafficking into the eye only after lymphocyte activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatila T. A., Geha R. S. Phosphorylation of T cell membrane proteins by activators of protein kinase C. J Immunol. 1988 Jun 15;140(12):4308–4314. [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., McKinnon A., Liversidge J., Forrester J. V. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Ophthalmol. 1993 Mar;77(3):171–175. doi: 10.1136/bjo.77.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Craig A., Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992 Jan;116(1):219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Forrester J. V. Duke-Elder Lecture: new concepts on the role of autoimmunity in the pathogenesis of uveitis. Eye (Lond) 1992;6(Pt 5):433–446. doi: 10.1038/eye.1992.93. [DOI] [PubMed] [Google Scholar]
- Hogg N. Roll, roll, roll your leucocyte gently down the vein.... Immunol Today. 1992 Apr;13(4):113–115. doi: 10.1016/0167-5699(92)90103-E. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Savage C. O., Pober J. S. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J Exp Med. 1990 May 1;171(5):1453–1467. doi: 10.1084/jem.171.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg J. F., Smeets E. F., Neefjes J. J., Shaffer M. A., Cinek T., Jeunhomme T. M., Ahern T. J., Buurman W. A. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992 Dec;77(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- Liversidge J., McKay D., Mullen G., Forrester J. V. Retinal pigment epithelial cells modulate lymphocyte function at the blood-retina barrier by autocrine PGE2 and membrane-bound mechanisms. Cell Immunol. 1993 Jul;149(2):315–330. doi: 10.1006/cimm.1993.1158. [DOI] [PubMed] [Google Scholar]
- Liversidge J., Sewell H. F., Forrester J. V. Interactions between lymphocytes and cells of the blood-retina barrier: mechanisms of T lymphocyte adhesion to human retinal capillary endothelial cells and retinal pigment epithelial cells in vitro. Immunology. 1990 Nov;71(3):390–396. [PMC free article] [PubMed] [Google Scholar]
- Male D., Pyrce G., Hughes C., Lantos P. Lymphocyte migration into brain modelled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell Immunol. 1990 Apr 15;127(1):1–11. doi: 10.1016/0008-8749(90)90109-5. [DOI] [PubMed] [Google Scholar]
- Mayerson P. L., Hall M. O., Clark V., Abrams T. An improved method for isolation and culture of rat retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1599–1609. [PubMed] [Google Scholar]
- McIntosh L. C., Muckersie L., Forrester J. V. Retinal capillary endothelial cells prefer different substrates for growth and migration. Tissue Cell. 1988;20(2):193–209. doi: 10.1016/0040-8166(88)90041-9. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Immunologic interactions of T lymphocytes with vascular endothelium. Adv Immunol. 1991;50:261–302. doi: 10.1016/s0065-2776(08)60827-5. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Sewell H. F., Milton J. I., MacKenzie R., King D. J., Dawson A. A., Lessels S. E., Bennett B., Davidson R. L., Walker F. Immunophenotyping of leukaemias by flow cytometry and APAAP: a two-year comparative analysis in hospital practice. Dis Markers. 1988 Oct-Dec;6(4):221–229. [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Tanaka T., Miyasaka M. Molecular mechanisms underlying lymphocyte recirculation. II. Differential regulation of LFA-1 in the interaction between lymphocytes and high endothelial cells. Eur J Immunol. 1991 Mar;21(3):855–858. doi: 10.1002/eji.1830210351. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Welder C. A., Lee D. H., Takei F. Inhibition of cell adhesion by microspheres coated with recombinant soluble intercellular adhesion molecule-1. J Immunol. 1993 Mar 15;150(6):2203–2210. [PubMed] [Google Scholar]
- Whitcup S. M., DeBarge L. R., Caspi R. R., Harning R., Nussenblatt R. B., Chan C. C. Monoclonal antibodies against ICAM-1 (CD54) and LFA-1 (CD11a/CD18) inhibit experimental autoimmune uveitis. Clin Immunol Immunopathol. 1993 May;67(2):143–150. doi: 10.1006/clin.1993.1057. [DOI] [PubMed] [Google Scholar]



