Abstract
In this report we show that rat retinal pigment epithelial (RPE) cells express an inducible form of nitric oxide synthase (iNOS) and secrete high levels of nitric oxide (NO.) when co-cultured with activated lymphocytes. We have previously shown that cultured rat RPE cells suppress syngeneic lymphocyte proliferation, an effect attributed to prostaglandin E2 (PGE2) secretion by the RPE cells. However supernatants from such co-cultures were also found to contain high levels of nitrite (NO2-), the stable end-product of NO. synthesis. RPE cell secretion of NO. was stimulated by the cytokines interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha), an effect enhanced by endotoxin [lipopolysaccharide (LPS)], reduced by the competitive inhibitor of L-arginine metabolism, NG-monomethyl-L-arginine (L-NMMA) and inhibited by cycloheximide. These effects were dose dependent. Using reverse transcription (RT)/PCR a product of 1398 bp was amplified which showed sequence identity with iNOS cloned from rat vascular smooth muscle. Northern blot analysis of total RNA extracted from rat RPE before and after cytokine stimulation showed induction of a 4.5 kb (kilobase) transcript which hybridized with a 1398 bp (base pair) polymerase chain reaction (PCR)-generated cDNA probe derived from the sequence of rat RPE cell iNOS. These results indicate RPE cells express an inducible form of nitric oxide synthase (NOS) and that high levels of NO. may be produced locally in the eye by the RPE in the presence of activated lymphocytes. Given the cytostatic and cytotoxic properties of this molecule, NO. may play an important role as an inducible mediator of immunosuppressive mechanisms within the microenvironment of the eye at the site of lymphocyte activation.
Full text
PDF

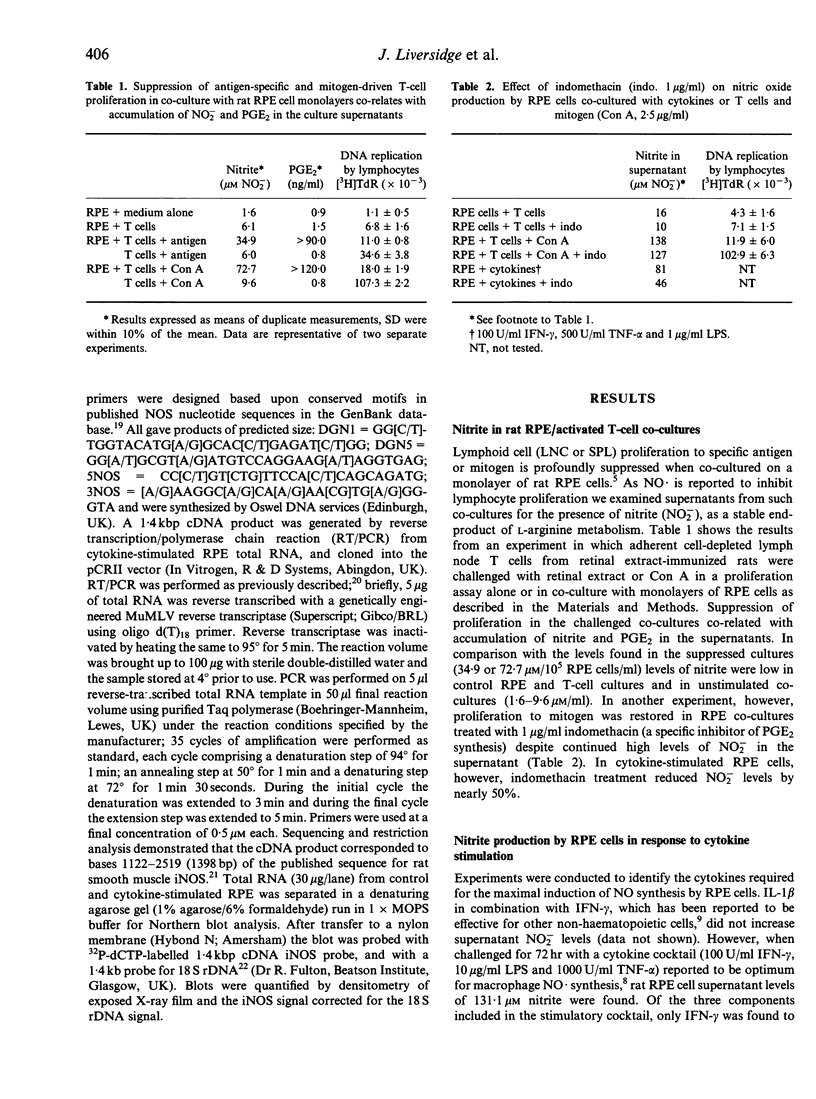
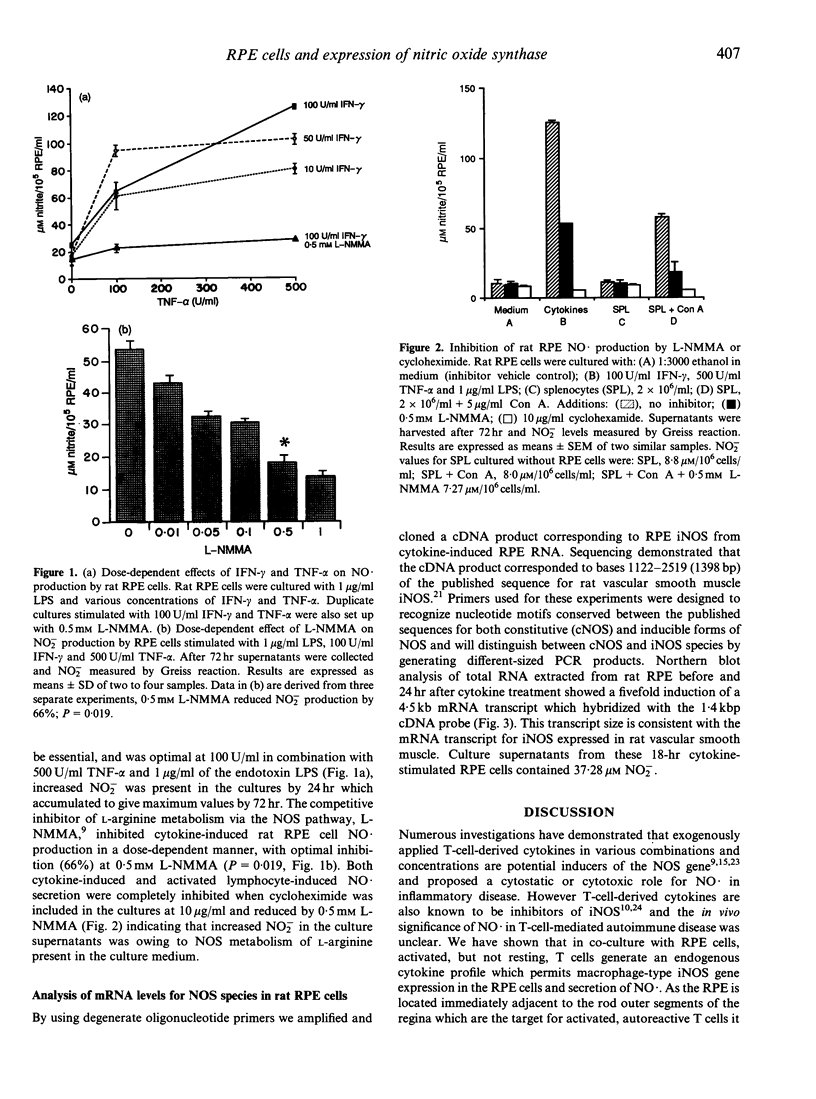
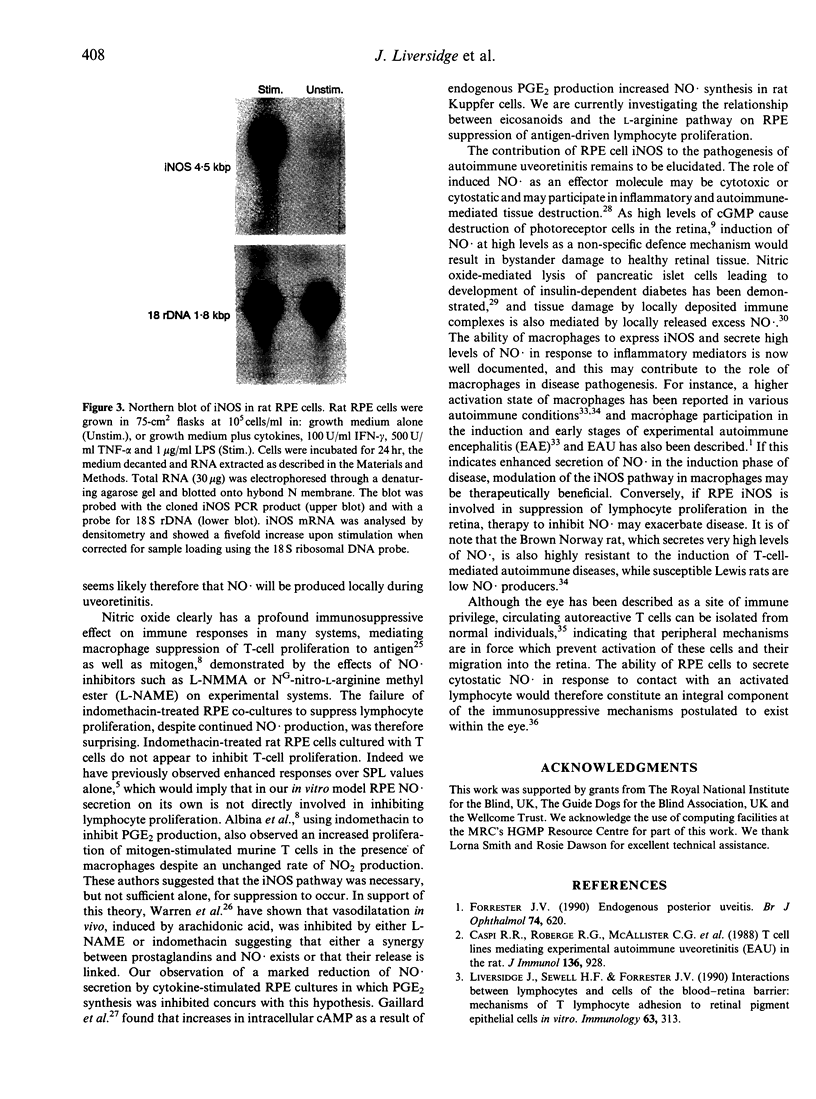

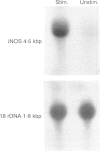
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Baird K. S., Crossan J. F., Ralston S. H. Abnormal growth factor and cytokine expression in Dupuytren's contracture. J Clin Pathol. 1993 May;46(5):425–428. doi: 10.1136/jcp.46.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan G., Barrett K., Turner M., Chantry D., Maini R. N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988 Sep;73(3):449–455. [PMC free article] [PubMed] [Google Scholar]
- Burks C., Cassidy M., Cinkosky M. J., Cumella K. E., Gilna P., Hayden J. E., Keen G. M., Kelley T. A., Kelly M., Kristofferson D. GenBank. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2221–2225. doi: 10.1093/nar/19.suppl.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., McDaniel M. L. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992 Aug;41(8):897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., McKinnon A., Liversidge J., Forrester J. V. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Ophthalmol. 1993 Mar;77(3):171–175. doi: 10.1136/bjo.77.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rushford C. L., Dorney D. J., Wilson G. N., Schmickel R. D. Structure and variation of human ribosomal DNA: molecular analysis of cloned fragments. Gene. 1981 Dec;16(1-3):1–9. doi: 10.1016/0378-1119(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Forrester J. V. Endogenous posterior uveitis. Br J Ophthalmol. 1990 Oct;74(10):620–623. doi: 10.1136/bjo.74.10.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester J. V., McMenamin P. G., Holthouse I., Lumsden L., Liversidge J. Localization and characterization of major histocompatibility complex class II-positive cells in the posterior segment of the eye: implications for induction of autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):64–77. [PubMed] [Google Scholar]
- Fu Y., Blankenhorn E. P. Nitric oxide-induced anti-mitogenic effects in high and low responder rat strains. J Immunol. 1992 Apr 1;148(7):2217–2222. [PubMed] [Google Scholar]
- Gaillard T., Mülsch A., Klein H., Decker K. Regulation by prostaglandin E2 of cytokine-elicited nitric oxide synthesis in rat liver macrophages. Biol Chem Hoppe Seyler. 1992 Sep;373(9):897–902. doi: 10.1515/bchm3.1992.373.2.897. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., Hieny S., James S. L., Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992 Oct;22(10):2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- Goureau O., Lepoivre M., Becquet F., Courtois Y. Differential regulation of inducible nitric oxide synthase by fibroblast growth factors and transforming growth factor beta in bovine retinal pigmented epithelial cells: inverse correlation with cellular proliferation. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4276–4280. doi: 10.1073/pnas.90.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hirose S., Tanaka T., Nussenblatt R. B., Palestine A. G., Wiggert B., Redmond T. M., Chader G. J., Gery I. Lymphocyte responses to retinal-specific antigens in uveitis patients and healthy subjects. Curr Eye Res. 1988 Apr;7(4):393–402. doi: 10.3109/02713688809031789. [DOI] [PubMed] [Google Scholar]
- Hoffman R. A., Langrehr J. M., Billiar T. R., Curran R. D., Simmons R. L. Alloantigen-induced activation of rat splenocytes is regulated by the oxidative metabolism of L-arginine. J Immunol. 1990 Oct 1;145(7):2220–2226. [PubMed] [Google Scholar]
- Huitinga I., van Rooijen N., de Groot C. J., Uitdehaag B. M., Dijkstra C. D. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990 Oct 1;172(4):1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992 May;13(5):157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Severn A., Millott S., Schmidt J., Salter M., Moncada S. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991 Oct;21(10):2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- Liversidge J., McKay D., Mullen G., Forrester J. V. Retinal pigment epithelial cells modulate lymphocyte function at the blood-retina barrier by autocrine PGE2 and membrane-bound mechanisms. Cell Immunol. 1993 Jul;149(2):315–330. doi: 10.1006/cimm.1993.1158. [DOI] [PubMed] [Google Scholar]
- Liversidge J., Sewell H. F., Thomson A. W., Forrester J. V. Lymphokine-induced MHC class II antigen expression on cultured retinal pigment epithelial cells and the influence of cyclosporin A. Immunology. 1988 Feb;63(2):313–317. [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mulligan M. S., Moncada S., Ward P. A. Protective effects of inhibitors of nitric oxide synthase in immune complex-induced vasculitis. Br J Pharmacol. 1992 Dec;107(4):1159–1162. doi: 10.1111/j.1476-5381.1992.tb13423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nunokawa Y., Ishida N., Tanaka S. Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Feb 26;191(1):89–94. doi: 10.1006/bbrc.1993.1188. [DOI] [PubMed] [Google Scholar]
- Percopo C. M., Hooks J. J., Shinohara T., Caspi R., Detrick B. Cytokine-mediated activation of a neuronal retinal resident cell provokes antigen presentation. J Immunol. 1990 Dec 15;145(12):4101–4107. [PubMed] [Google Scholar]
- Products & materials. Science. 1992 Apr 10;256(5054):255–255. doi: 10.1126/science.256.5054.255. [DOI] [PubMed] [Google Scholar]
- Punjabi C. J., Laskin D. L., Heck D. E., Laskin J. D. Production of nitric oxide by murine bone marrow cells. Inverse correlation with cellular proliferation. J Immunol. 1992 Sep 15;149(6):2179–2184. [PubMed] [Google Scholar]
- Rothe H., Fehsel K., Kolb H. Tumour necrosis factor alpha production is upregulated in diabetes prone BB rats. Diabetologia. 1990 Sep;33(9):573–575. doi: 10.1007/BF00404147. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Coughlan M. L., Williams T. J. Endotoxin-induced vasodilatation in anaesthetized rat skin involves nitric oxide and prostaglandin synthesis. Br J Pharmacol. 1992 Aug;106(4):953–957. doi: 10.1111/j.1476-5381.1992.tb14441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]