Abstract
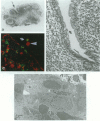
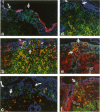
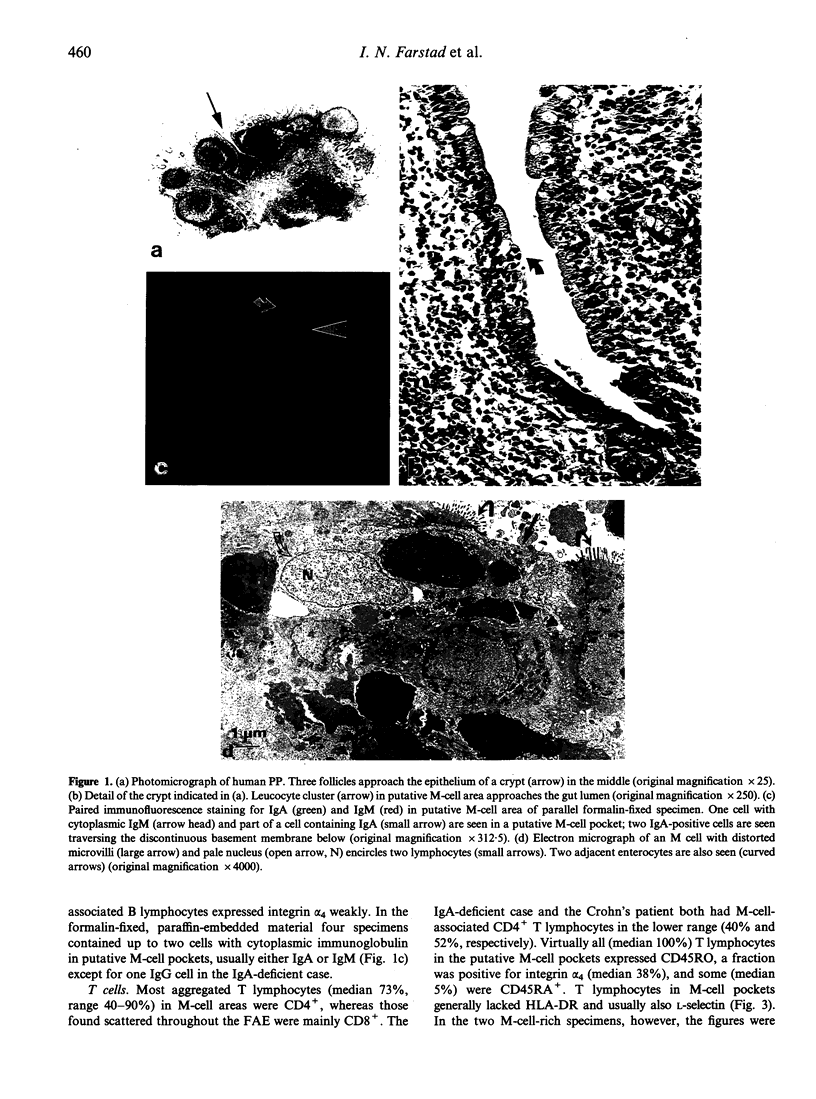
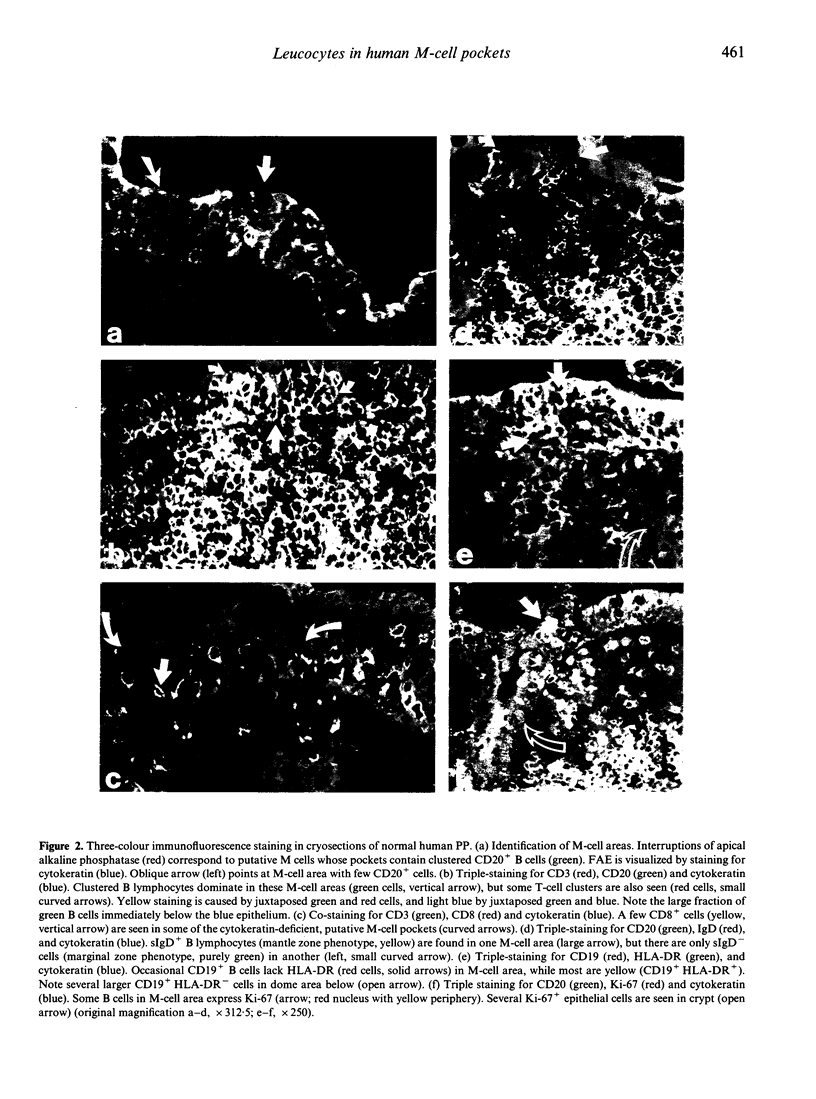
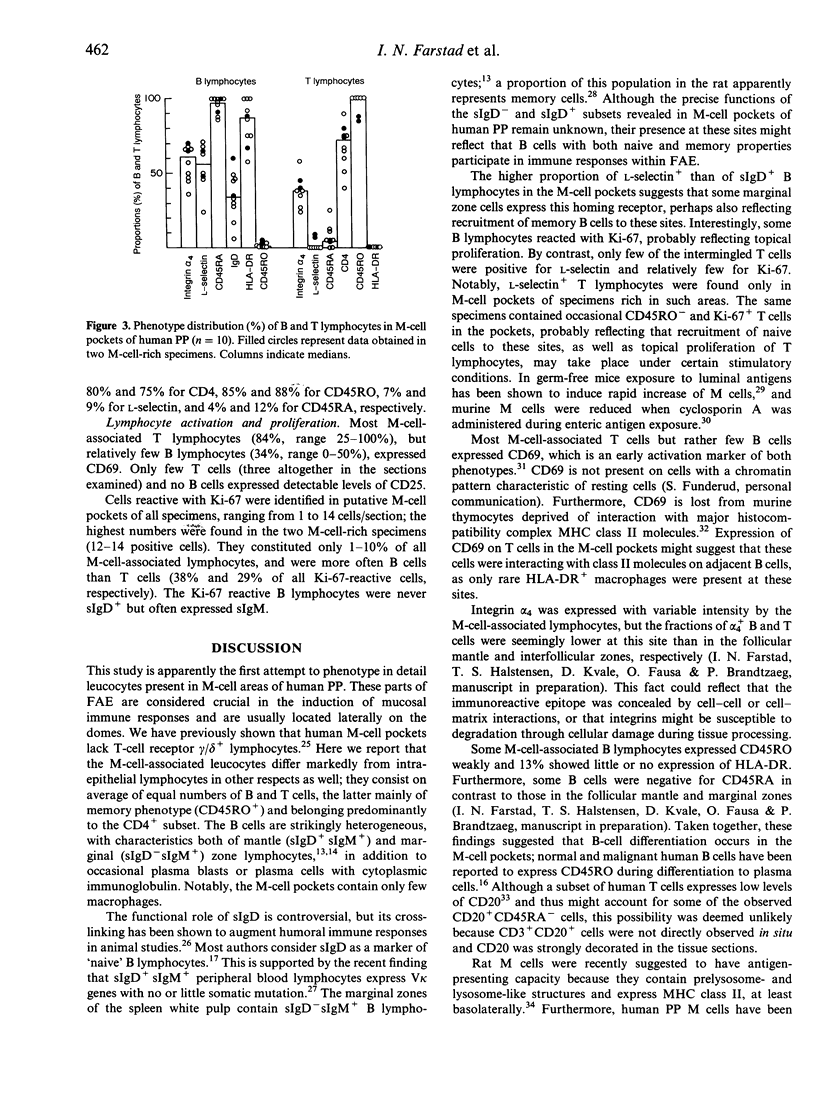
The specialized M cells in the follicle-associated epithelium (FAE) of Peyer's patches (PP) represent an intimate interphase between luminal antigens and gut-associated lymphoid tissue (GALT). M cells form pockets that contain clusters of leucocytes probably involved in the first encounter with antigens from the gut lumen. Three-colour immunofluorescence in situ phenotyping of these leucocytes in humans revealed about equal numbers of B (CD19/20+) and T(CD3+) lymphocytes, the latter mainly CD4+ (median 73%, range 40-90%), but relatively few macrophages (CD68+). Most B cells (90%) were positive for surface IgM (sIgM) and often co-expressed sIgD (median 34%, range 6-60%). Occasional B cells (median 2%) did not express CD45RA (range 0-15%) and 13% virtually lacked HLA-DR (range 0-40%). Some B and T lymphocytes expressed the nuclear proliferation marker Ki-67 (range 1-10%). The M-cell pockets also contained occasional cells with cytoplasmic IgA or IgM. These sites thus contained a heterogeneous B-cell population with features of both follicular mantle (sIgD+ sIgM+) and marginal zone (sIgD- sIgM+) B lymphocytes. Adjacent T lymphocytes were generally of the memory phenotype (CD45RO+). Our findings suggest that the M-cell-associated B lymphocytes represent local extensions of B-cell follicles towards the gut lumen, developed topically to facilitate antigen presentation and diversification of mucosal immune responses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan C. H., Mendrick D. L., Trier J. S. Rat intestinal M cells contain acidic endosomal-lysosomal compartments and express class II major histocompatibility complex determinants. Gastroenterology. 1993 Mar;104(3):698–708. doi: 10.1016/0016-5085(93)91004-2. [DOI] [PubMed] [Google Scholar]
- Amerongen H. M., Weltzin R., Mack J. A., Winner L. S., 3rd, Michetti P., Apter F. M., Kraehenbuhl J. P., Neutra M. R. M cell-mediated antigen transport and monoclonal IgA antibodies for mucosal immune protection. Ann N Y Acad Sci. 1992;664:18–26. doi: 10.1111/j.1749-6632.1992.tb39745.x. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Rousset F. Human B lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol. 1992;52:125–262. doi: 10.1016/s0065-2776(08)60876-7. [DOI] [PubMed] [Google Scholar]
- Bennett K., Levine T., Ellis J. S., Peanasky R. J., Samloff I. M., Kay J., Chain B. M. Antigen processing for presentation by class II major histocompatibility complex requires cleavage by cathepsin E. Eur J Immunol. 1992 Jun;22(6):1519–1524. doi: 10.1002/eji.1830220626. [DOI] [PubMed] [Google Scholar]
- Berlin C., Berg E. L., Briskin M. J., Andrew D. P., Kilshaw P. J., Holzmann B., Weissman I. L., Hamann A., Butcher E. C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993 Jul 16;74(1):185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- Beverley P. Immunological memory in T cells. Curr Opin Immunol. 1991 Jun;3(3):355–360. doi: 10.1016/0952-7915(91)90038-3. [DOI] [PubMed] [Google Scholar]
- Bjerke K., Brandtzaeg P., Fausa O. T cell distribution is different in follicle-associated epithelium of human Peyer's patches and villous epithelium. Clin Exp Immunol. 1988 Nov;74(2):270–275. [PMC free article] [PubMed] [Google Scholar]
- Bjerke K., Brandtzaeg P. Lack of relation between expression of HLA-DR and secretory component (SC) in follicle-associated epithelium of human Peyer's patches. Clin Exp Immunol. 1988 Mar;71(3):502–507. [PMC free article] [PubMed] [Google Scholar]
- Brändle D., Müller S., Müller C., Hengartner H., Pircher H. Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur J Immunol. 1994 Jan;24(1):145–151. doi: 10.1002/eji.1830240122. [DOI] [PubMed] [Google Scholar]
- Bye W. A., Allan C. H., Trier J. S. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology. 1984 May;86(5 Pt 1):789–801. [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak T. H., Owen R. L. Differential distribution of lymphocytes and accessory cells in mouse Peyer's patches. Anat Rec. 1986 Jun;215(2):144–152. doi: 10.1002/ar.1092150208. [DOI] [PubMed] [Google Scholar]
- Ermak T. H., Steger H. J., Pappo J. Phenotypically distinct subpopulations of T cells in domes and M-cell pockets of rabbit gut-associated lymphoid tissues. Immunology. 1990 Dec;71(4):530–537. [PMC free article] [PubMed] [Google Scholar]
- Farstad I. N., Halstensen T. S., Fausa O., Brandtzaeg P. Do human Peyer's patches contribute to the intestinal intraepithelial gamma/delta T-cell population? Scand J Immunol. 1993 Nov;38(5):451–458. doi: 10.1111/j.1365-3083.1993.tb02587.x. [DOI] [PubMed] [Google Scholar]
- Finkelham F. D., Woods V. L., Wilburn S. B., Mond J. J., Stein K. E., Berning A., Scher I. Augmentation of in vitro humoral immune responses in the mouse by an antibody to IgD. J Exp Med. 1980 Sep 1;152(3):493–506. doi: 10.1084/jem.152.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi G., Cornaggia M., Capella C., Fiocca R., Bosi F., Solcia E., Samloff I. M. Cathepsin E in follicle associated epithelium of intestine and tonsils: localization to M cells and possible role in antigen processing. Histochemistry. 1993 Mar;99(3):201–211. doi: 10.1007/BF00269138. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Brandtzaeg P. Intraepithelial T cells of the TcR gamma/delta+ CD8- and V delta 1/J delta 1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989 Dec;30(6):665–672. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Hamann A., Andrew D. P., Jablonski-Westrich D., Holzmann B., Butcher E. C. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994 Apr 1;152(7):3282–3293. [PubMed] [Google Scholar]
- Hamann A., Jablonski-Westrich D., Jonas P., Thiele H. G. Homing receptors reexamined: mouse LECAM-1 (MEL-14 antigen) is involved in lymphocyte migration into gut-associated lymphoid tissue. Eur J Immunol. 1991 Dec;21(12):2925–2929. doi: 10.1002/eji.1830211205. [DOI] [PubMed] [Google Scholar]
- Hara T., Jung L. K., Bjorndahl J. M., Fu S. M. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986 Dec 1;164(6):1988–2005. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultin L. E., Hausner M. A., Hultin P. M., Giorgi J. V. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14(2):196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- Jarry A., Robaszkiewicz M., Brousse N., Potet F. Immune cells associated with M cells in the follicle-associated epithelium of Peyer's patches in the rat. An electron- and immuno-electron-microscopic study. Cell Tissue Res. 1989 Feb;255(2):293–298. doi: 10.1007/BF00224111. [DOI] [PubMed] [Google Scholar]
- Jensen G. S., Poppema S., Mant M. J., Pilarski L. M. Transition in CD45 isoform expression during differentiation of normal and abnormal B cells. Int Immunol. 1989;1(3):229–236. doi: 10.1093/intimm/1.3.229. [DOI] [PubMed] [Google Scholar]
- Kanof M. E., Strober W., Fiocchi C., Zeitz M., James S. P. CD4 positive Leu-8 negative helper-inducer T cells predominate in the human intestinal lamina propria. J Immunol. 1988 Nov 1;141(9):3029–3036. [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993 Dec;23(12):3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Zhang J., Lane P. J., Chan E. Y., MacLennan I. C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991 Dec;21(12):2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Janeway C. A., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993 Apr;14(4):151–154. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Nagura H., Ohtani H., Masuda T., Kimura M., Nakamura S. HLA-DR expression on M cells overlying Peyer's patches is a common feature of human small intestine. Acta Pathol Jpn. 1991 Nov;41(11):818–823. doi: 10.1111/j.1440-1827.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Pulford K. A., Sipos A., Cordell J. L., Stross W. P., Mason D. Y. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973–980. doi: 10.1093/intimm/2.10.973. [DOI] [PubMed] [Google Scholar]
- Savidge T. C., Smith M. W., James P. S., Aldred P. Salmonella-induced M-cell formation in germ-free mouse Peyer's patch tissue. Am J Pathol. 1991 Jul;139(1):177–184. [PMC free article] [PubMed] [Google Scholar]
- Shvartsman Y. S., Agranovskaya E. N., Zykov M. P. Formation of secretory and circulating antibodies after immunization with live and inactivated influenza virus vaccines. J Infect Dis. 1977 May;135(5):697–705. doi: 10.1093/infdis/135.5.697. [DOI] [PubMed] [Google Scholar]
- Siciński P., Rowiński J., Warchoł J. B., Jarzabek Z., Gut W., Szczygieł B., Bielecki K., Koch G. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990 Jan;98(1):56–58. doi: 10.1016/0016-5085(90)91290-m. [DOI] [PubMed] [Google Scholar]
- Spencer J., Finn T., Isaacson P. G. Human Peyer's patches: an immunohistochemical study. Gut. 1986 Apr;27(4):405–410. doi: 10.1136/gut.27.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Finn T., Pulford K. A., Mason D. Y., Isaacson P. G. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol. 1985 Dec;62(3):607–612. [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Wigley FM Small P. A., Jr Specificity of respiratory secretion antibody against influenza virus. J Immunol. 1970 Dec;105(6):1477–1483. [PubMed] [Google Scholar]