Abstract
The four subclasses of IgG have different biological activities associated with their Fc regions. Fc gamma receptors on leucocytes (Fc gamma R) mediate binding and phagocytosis of opsonized particles. Two structurally and functionally distinct allelic polymorphisms of the Fc gamma R have been defined: the H/R131 forms of Fc gamma RIIa (CD32), and the neutrophil antigen 1 (NA1)/NA2 forms of Fc gamma RIIIb (CD16). In this study the activities of allotypes of CD16 are analysed with antibacterial IgG subclass antibodies and with IgG1 and IgG3 anti-Rhesus D, and the activities of CD32 with IgG1 and IgG3 anti-Rhesus D. With respect to the allotypes of CD16, polymorphonuclear leucocytes (PMN) homozygous for Fc gamma RIIb-NA2 exhibited a lower (21-25%) IgG1-mediated phagocytosis of Staphylococcus aureus strain Wood (STAW), Haemophilus influenzae type b (Hib), and Neisseria meningitidis group B (NMen) than IIIb-NA1 PMN. The difference was apparent only when the micro-organisms were opsonized in the absence of complement, and was furthermore enhanced (34-52%) upon blockade of Fc gamma RIIa. In addition, monoclonal IgG3 anti-D-mediated rosette formation and phagocytosis was consistently found to be lower (16%) with Fc gamma RIIIb-NA2 than with IIIb-NA1 PMN. For the allotypes of CD32 we now show that IgG3 anti-D sensitized erythrocytes formed more (50%) rosettes and were phagocytosed at a higher rate with PMN carrying Fc gamma RIIa-H131 than with PMN carrying IIa-R131. Heterozygous Fc gamma RIIa-H/R131 PMN exhibited intermediate phagocytic activity in this respect. This study illustrates a critical role of Fc gamma R allotypes in functional interactions with biologically relevant IgG subclass antibodies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoun G. R., Longenecker B. M., Zipf T. F. Comparison of the 40 kDa hematopoietic cell antigens bound by monoclonal antibodies IV.3, 41H.16 and KB61. Mol Immunol. 1989 Mar;26(3):333–338. doi: 10.1016/0161-5890(89)90088-6. [DOI] [PubMed] [Google Scholar]
- Blasini A. M., Stekman I. L., Leon-Ponte M., Caldera D., Rodriguez M. A. Increased proportion of responders to a murine anti-CD3 monoclonal antibody of the IgG1 class in patients with systemic lupus erythematosus (SLE). Clin Exp Immunol. 1993 Dec;94(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- Bredius R. G., Driedijk P. C., Schouten M. F., Weening R. S., Out T. A. Complement activation by polyclonal immunoglobulin G1 and G2 antibodies against Staphylococcus aureus, Haemophilus influenzae type b, and tetanus toxoid. Infect Immun. 1992 Nov;60(11):4838–4847. doi: 10.1128/iai.60.11.4838-4847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
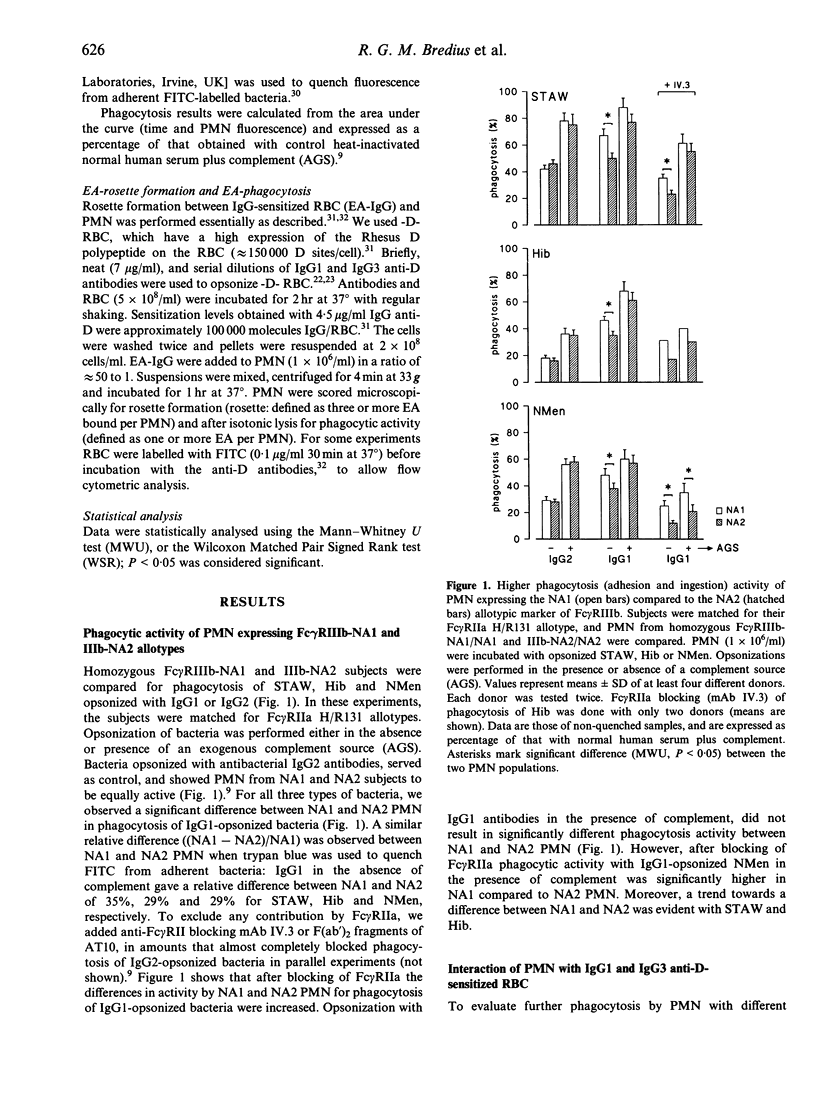
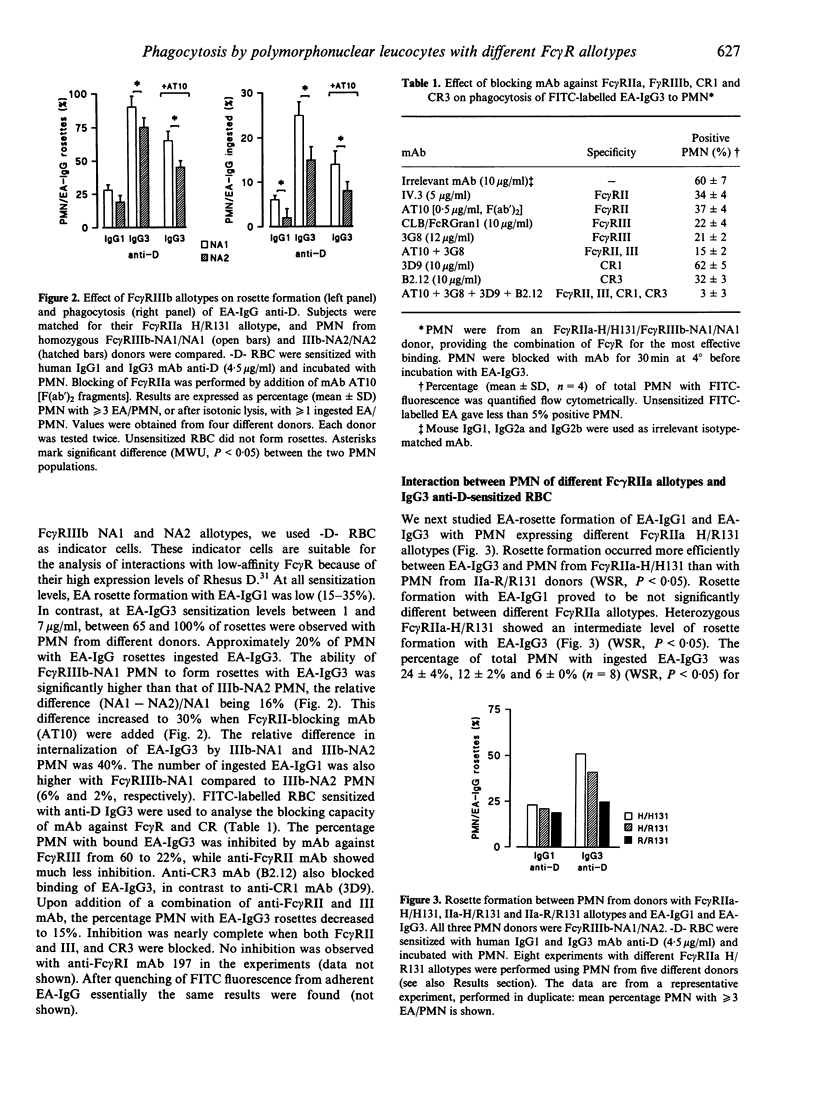
- Bredius R. G., de Vries C. E., Troelstra A., van Alphen L., Weening R. S., van de Winkel J. G., Out T. A. Phagocytosis of Staphylococcus aureus and Haemophilus influenzae type B opsonized with polyclonal human IgG1 and IgG2 antibodies. Functional hFc gamma RIIa polymorphism to IgG2. J Immunol. 1993 Aug 1;151(3):1463–1472. [PubMed] [Google Scholar]
- Brunkhorst B. A., Strohmeier G., Lazzari K., Weil G., Melnick D., Fleit H. B., Simons E. R. Differential roles of Fc gamma RII and Fc gamma RIII in immune complex stimulation of human neutrophils. J Biol Chem. 1992 Oct 15;267(29):20659–20666. [PubMed] [Google Scholar]
- Burton D. R., Woof J. M. Human antibody effector function. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- Falconer A. E., Carson R., Johnstone R., Bird P., Kehoe M., Calvert J. E. Distinct IgG1 and IgG3 subclass responses to two streptococcal protein antigens in man: analysis of antibodies to streptolysin O and M protein using standardized subclass-specific enzyme-linked immunosorbent assays. Immunology. 1993 May;79(1):89–94. [PMC free article] [PubMed] [Google Scholar]
- Fijen C. A., Bredius R. G., Kuijper E. J. Polymorphism of IgG Fc receptors in meningococcal disease. Ann Intern Med. 1993 Oct 1;119(7 Pt 1):636–636. doi: 10.7326/0003-4819-119-7_part_1-199310010-00026. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin E. J., Brown M. F., Anderson C. L., Zipf T. F., Guyre P. M. The monoclonal antibody 41H16 detects the Leu 4 responder form of human Fc gamma RII. J Immunol. 1990 Mar 1;144(5):1817–1822. [PubMed] [Google Scholar]
- Graham I. L., Gresham H. D., Brown E. J. An immobile subset of plasma membrane CD11b/CD18 (Mac-1) is involved in phagocytosis of targets recognized by multiple receptors. J Immunol. 1989 Apr 1;142(7):2352–2358. [PubMed] [Google Scholar]
- Greenman J., Tutt A. L., George A. J., Pulford K. A., Stevenson G. T., Glennie M. J. Characterization of a new monoclonal anti-Fc gamma RII antibody, AT10, and its incorporation into a bispecific F(ab')2 derivative for recruitment of cytotoxic effectors. Mol Immunol. 1991 Nov;28(11):1243–1254. doi: 10.1016/0161-5890(91)90011-8. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Graziano R. F., Vance B. A., Morganelli P. M., Fanger M. W. Monoclonal antibodies that bind to distinct epitopes on Fc gamma RI are able to trigger receptor function. J Immunol. 1989 Sep 1;143(5):1650–1655. [PubMed] [Google Scholar]
- Hadley A. G., Zupanska B., Kumpel B. M., Leader K. A. The functional activity of Fc gamma RII and Fc gamma RIII on subsets of human lymphocytes. Immunology. 1992 Jul;76(3):446–451. [PMC free article] [PubMed] [Google Scholar]
- Huizinga T. W., Kerst M., Nuyens J. H., Vlug A., von dem Borne A. E., Roos D., Tetteroo P. A. Binding characteristics of dimeric IgG subclass complexes to human neutrophils. J Immunol. 1989 Apr 1;142(7):2359–2364. [PubMed] [Google Scholar]
- Huizinga T. W., Kleijer M., Tetteroo P. A., Roos D., von dem Borne A. E. Biallelic neutrophil Na-antigen system is associated with a polymorphism on the phospho-inositol-linked Fc gamma receptor III (CD16). Blood. 1990 Jan 1;75(1):213–217. [PubMed] [Google Scholar]
- Huizinga T. W., Kuijpers R. W., Kleijer M., Schulpen T. W., Cuypers H. T., Roos D., von dem Borne A. E. Maternal genomic neutrophil FcRIII deficiency leading to neonatal isoimmune neutropenia. Blood. 1990 Nov 15;76(10):1927–1932. [PubMed] [Google Scholar]
- Kimberly R. P., Ahlstrom J. W., Click M. E., Edberg J. C. The glycosyl phosphatidylinositol-linked Fc gamma RIIIPMN mediates transmembrane signaling events distinct from Fc gamma RII. J Exp Med. 1990 Apr 1;171(4):1239–1255. doi: 10.1084/jem.171.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpel B. M., Hadley A. G. Functional interactions of red cells sensitized by IgG1 and IgG3 human monoclonal anti-D with enzyme-modified human monocytes and FcR-bearing cell lines. Mol Immunol. 1990 Mar;27(3):247–256. doi: 10.1016/0161-5890(90)90137-o. [DOI] [PubMed] [Google Scholar]
- Kumpel B. M., Leader K. A., Merry A. H., Hadley A. G., Poole G. D., Blancher A., Goossens D., Hughes-Jones N. C., Bradley B. A. Heterogeneity in the ability of IgG1 monoclonal anti-D to promote lymphocyte-mediated red cell lysis. Eur J Immunol. 1989 Dec;19(12):2283–2288. doi: 10.1002/eji.1830191216. [DOI] [PubMed] [Google Scholar]
- Looney R. J., Abraham G. N., Anderson C. L. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986 Mar 1;136(5):1641–1647. [PubMed] [Google Scholar]
- Parren P. W., Warmerdam P. A., Boeije L. C., Arts J., Westerdaal N. A., Vlug A., Capel P. J., Aarden L. A., van de Winkel J. G. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992 Oct;90(4):1537–1546. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Ruths S., Driedijk P. C., Weening R. S., Out T. A. ELISA procedures for the measurement of IgG subclass antibodies to bacterial antigens. J Immunol Methods. 1991 Jun 24;140(1):67–78. doi: 10.1016/0022-1759(91)90127-2. [DOI] [PubMed] [Google Scholar]
- Sahlin S., Hed J., Rundquist I. Differentiation between attached and ingested immune complexes by a fluorescence quenching cytofluorometric assay. J Immunol Methods. 1983 May 27;60(1-2):115–124. doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- Salmon J. E., Brogle N. L., Edberg J. C., Kimberly R. P. Fc gamma receptor III induces actin polymerization in human neutrophils and primes phagocytosis mediated by Fc gamma receptor II. J Immunol. 1991 Feb 1;146(3):997–1004. [PubMed] [Google Scholar]
- Salmon J. E., Edberg J. C., Brogle N. L., Kimberly R. P. Allelic polymorphisms of human Fc gamma receptor IIA and Fc gamma receptor IIIB. Independent mechanisms for differences in human phagocyte function. J Clin Invest. 1992 Apr;89(4):1274–1281. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. E., Edberg J. C., Kimberly R. P. Fc gamma receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J Clin Invest. 1990 Apr;85(4):1287–1295. doi: 10.1172/JCI114566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford P. G., Nelson S. J., Palma A. T., Nahm M. H. Human antibodies to group A streptococcal carbohydrate. Ontogeny, subclass restriction, and clonal diversity. J Immunol. 1988 May 1;140(9):3200–3205. [PubMed] [Google Scholar]
- Tuijnman W. B., Van de Winkel J. G., Capel P. J. A flow cytometric rosetting assay for the analysis of IgG-Fc receptor interactions. J Immunol Methods. 1990 Mar 9;127(2):207–214. doi: 10.1016/0022-1759(90)90070-c. [DOI] [PubMed] [Google Scholar]
- Zhou M., Todd R. F., 3rd, van de Winkel J. G., Petty H. R. Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fc gamma receptor III on human neutrophils. Possible role of lectin-like interactions. J Immunol. 1993 Apr 1;150(7):3030–3041. [PubMed] [Google Scholar]
- van de Winkel J. G., Anderson C. L. Biology of human immunoglobulin G Fc receptors. J Leukoc Biol. 1991 May;49(5):511–524. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]
- van de Winkel J. G., Capel P. J. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993 May;14(5):215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]


