Abstract
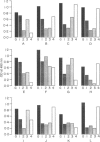
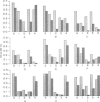
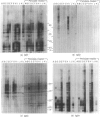
In areas endemic for schistosomiasis, there is great heterogeneity in antibody isotype responses to parasite antigens amongst infected individuals. At the population level, the isotype composition of antibody responses undergoes dynamic changes which are associated with the age of infected individuals. Here we examine the IgG subclass responses to Schistosoma mansoni eggs (soluble egg antigens; SEA) of infected individuals by immunoblot and ELISA. By controlled treatment of SEA-coated ELISA plates and immunoblot nitrocellular strips with sodium periodate, in order to oxidize terminal carbohydrate residues selectively, we were able to relate individuals subjects' isotype responses to the different antigens that they responded to, and to the presence of putative carbohydrate and peptide epitopes on those antigens. IgG2 responses were restricted strictly to sodium periodate-sensitive carbohydrate epitopes and antigens of relatively high molecular weight. These antigens were not usually recognized by other isotypes and, therefore, they were only recognized by individuals who had high levels of IgG2. IgG1 and IgG3 responses were directed against both carbohydrate and peptide epitopes, whereas IgG4 responses were restricted to periodate-resistant epitopes. This suggests that the fall in IgG2 responses, and reciprocal rise in IgG4 antibodies, seen in young children as their intensities of schistosome infection increase, is not the result of isotype switching, and that, if these two subclasses are involved in blocking immunity to schistosomiasis, they are operating independently.
Full text
PDF

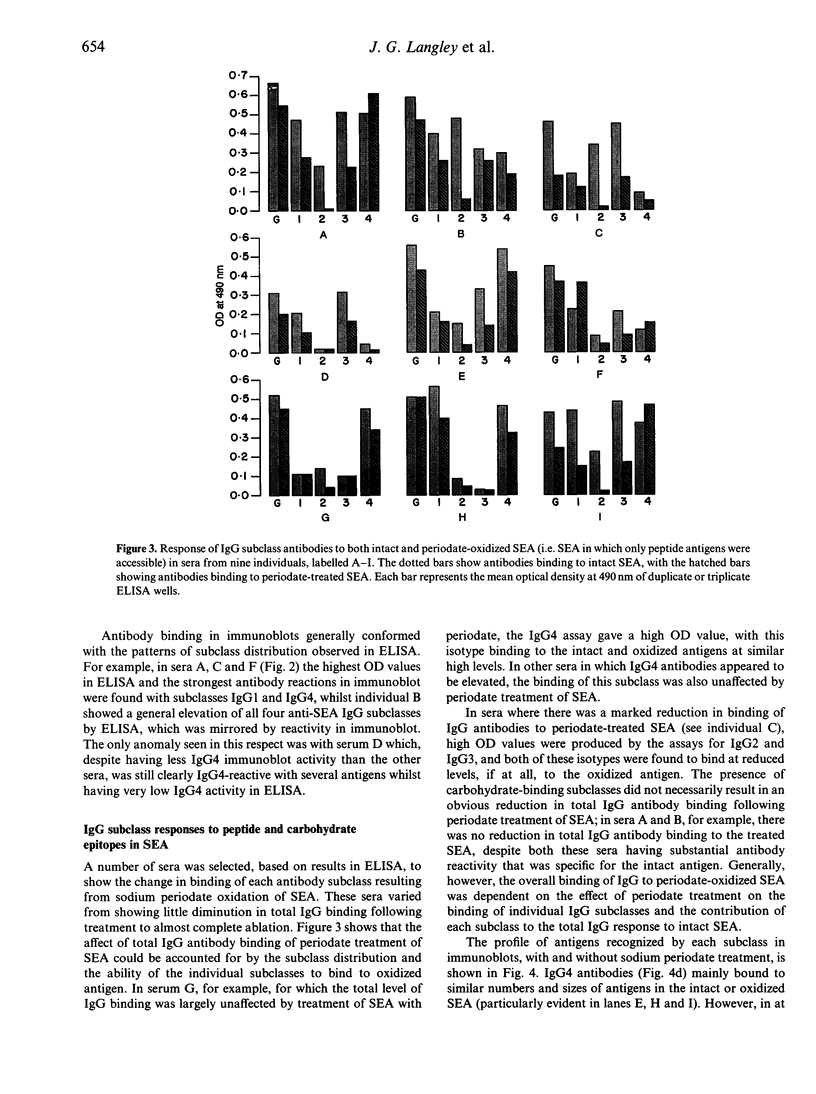
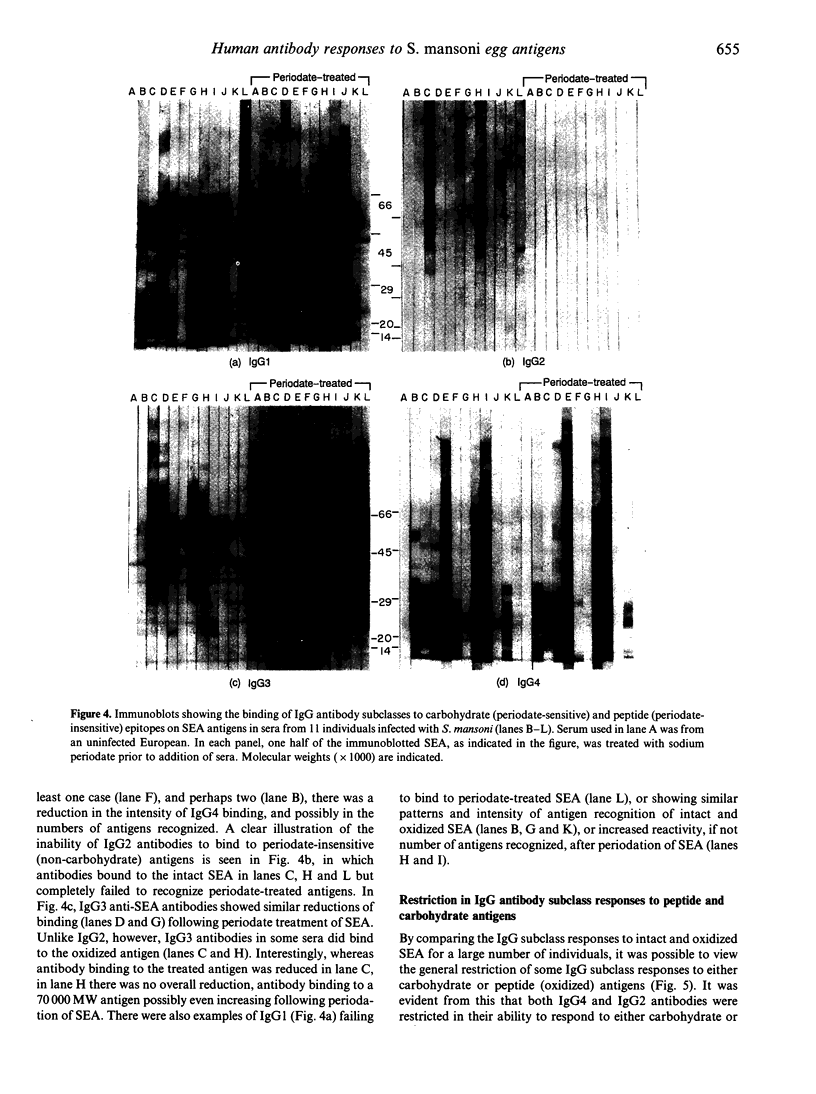
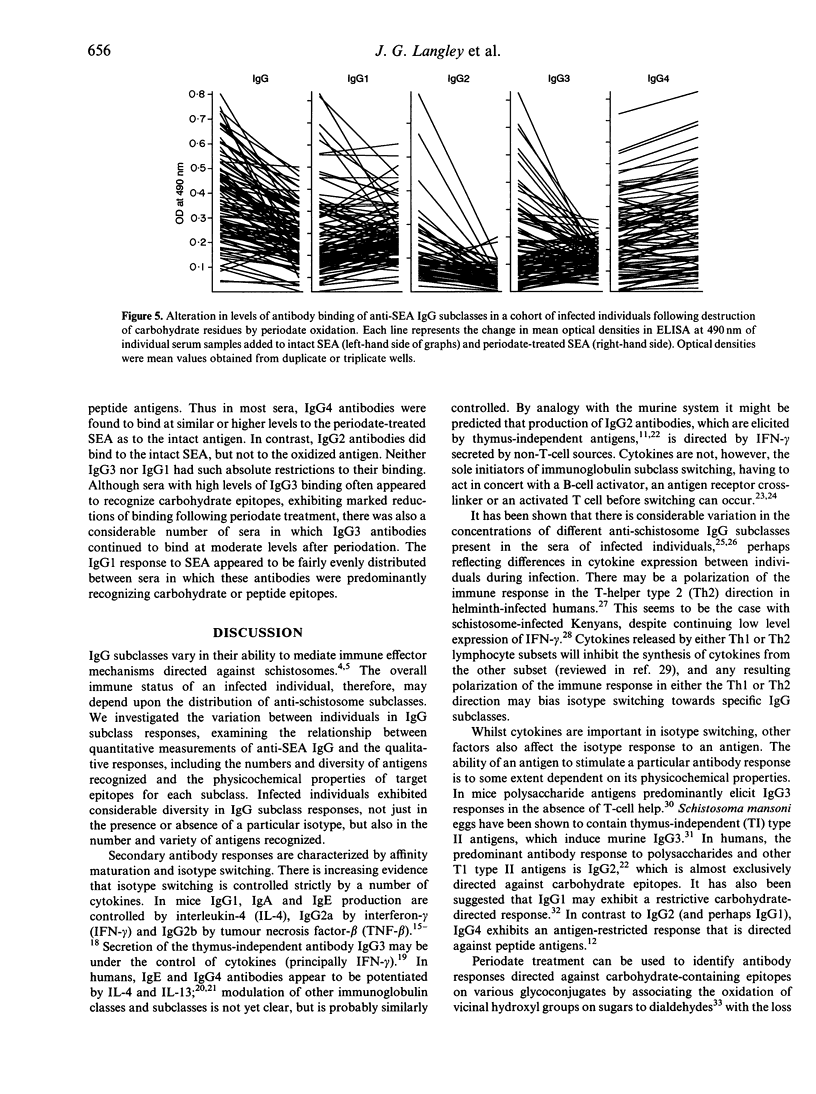


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boctor F. N., Peter J. B. IgG subclasses in human chronic schistosomiasis: over-production of schistosome-specific and non-specific IgG4. Clin Exp Immunol. 1990 Dec;82(3):574–578. doi: 10.1111/j.1365-2249.1990.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A., Dunne D., Fulford A., Capron M., Khalife J., Capron A., Koech D., Ouma J., Sturrock R. Immunity in human schistosomiasis mansoni: cross-reactive IgM and IgG2 anti-carbohydrate antibodies block the expression of immunity. Biochimie. 1988 Aug;70(8):1053–1063. doi: 10.1016/0300-9084(88)90268-4. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Reisfeld R. A., Varki A. P. O-acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984 Aug 24;225(4664):844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Demeure C. E., Rihet P., Abel L., Ouattara M., Bourgois A., Dessein A. J. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993 Oct;168(4):1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Bain J., Lillywhite J., Doenhoff M. J. The stage-, strain- and species-specificity of a Schistosoma mansoni egg antigen fraction (CEF6) with serodiagnostic potential. Trans R Soc Trop Med Hyg. 1984;78(4):460–470. doi: 10.1016/0035-9203(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Butterworth A. E., Fulford A. J., Kariuki H. C., Langley J. G., Ouma J. H., Capron A., Pierce R. J., Sturrock R. F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992 Jun;22(6):1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Grabowska A. M., Fulford A. J., Butterworth A. E., Sturrock R. F., Koech D., Ouma J. H. Human antibody responses to Schistosoma mansoni: the influence of epitopes shared between different life-cycle stages on the response to the schistosomulum. Eur J Immunol. 1988 Jan;18(1):123–131. doi: 10.1002/eji.1830180119. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Richardson B. A., Jones F. M., Clark M., Thorne K. J., Butterworth A. E. The use of mouse/human chimaeric antibodies to investigate the roles of different antibody isotypes, including IgA2, in the killing of Schistosoma mansoni schistosomula by eosinophils. Parasite Immunol. 1993 Mar;15(3):181–185. doi: 10.1111/j.1365-3024.1993.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Esser C., Radbruch A. Immunoglobulin class switching: molecular and cellular analysis. Annu Rev Immunol. 1990;8:717–735. doi: 10.1146/annurev.iy.08.040190.003441. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Urban J. F., Jr Cytokines: making the right choice. Parasitol Today. 1992 Sep;8(9):311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- Goodman D. J., Gaff C., Gerondakis S. The IL-4 induced increase in the frequency of resting murine splenic B cells expressing germline Ig heavy chain gamma 1 transcripts correlates with subsequent switching to IgG1. Int Immunol. 1993 Feb;5(2):199–208. doi: 10.1093/intimm/5.2.199. [DOI] [PubMed] [Google Scholar]
- Hagan P., Blumenthal U. J., Dunn D., Simpson A. J., Wilkins H. A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991 Jan 17;349(6306):243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983 Aug;71(2):231–251. [PubMed] [Google Scholar]
- Jassim A., Hassan K., Catty D. Antibody isotypes in human schistosomiasis mansoni. Parasite Immunol. 1987 Nov;9(6):627–650. doi: 10.1111/j.1365-3024.1987.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Kumararatne D. S. Selective IgG subclass deficiency: quantification and clinical relevance. Clin Exp Immunol. 1990 Sep;81(3):357–367. doi: 10.1111/j.1365-2249.1990.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalife J., Capron M., Capron A., Grzych J. M., Butterworth A. E., Dunne D. W., Ouma J. H. Immunity in human schistosomiasis mansoni. Regulation of protective immune mechanisms by IgM blocking antibodies. J Exp Med. 1986 Nov 1;164(5):1626–1640. doi: 10.1084/jem.164.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalife J., Dunne D. W., Richardson B. A., Mazza G., Thorne K. J., Capron A., Butterworth A. E. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni. J Immunol. 1989 Jun 15;142(12):4422–4427. [PubMed] [Google Scholar]
- Lal R. B., Dhawan R. R., Tarrand J. J., Ayoub E. M., Ottesen E. A. Lack of IgG4 antibody response to carbohydrate antigens in patients with lymphatic filariasis. Immunology. 1991 Oct;74(2):333–337. [PMC free article] [PubMed] [Google Scholar]
- Langley J. G., Dunne D. W. Temporal variation in the carbohydrate and peptide surface epitopes in antibody-dependent, eosinophil-mediated killing of Schistosoma mansoni schistosomula. Parasite Immunol. 1992 Mar;14(2):185–200. doi: 10.1111/j.1365-3024.1992.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Mahanty S., Abrams J. S., King C. L., Limaye A. P., Nutman T. B. Parallel regulation of IL-4 and IL-5 in human helminth infections. J Immunol. 1992 Jun 1;148(11):3567–3571. [PubMed] [Google Scholar]
- Mazza G., Dunne D. W., Butterworth A. E. Antibody isotype responses to the Schistosoma mansoni schistosomulum in the CBA/N mouse induced by different stages of the parasite life cycle. Parasite Immunol. 1990 Sep;12(5):529–543. doi: 10.1111/j.1365-3024.1990.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Nahm M., Stein K. E., Slack J., Zitron I., Paul W. E., Davie J. M. Immunoglobulin subclass-specific immunodeficiency in mice with an X-linked B-lymphocyte defect. J Exp Med. 1979 Apr 1;149(4):993–998. doi: 10.1084/jem.149.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen J., Aversa G., Cocks B. G., McKenzie A. N., Menon S., Zurawski G., de Waal Malefyt R., de Vries J. E. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihet P., Demeure C. E., Bourgois A., Prata A., Dessein A. J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991 Nov;21(11):2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- Roberts M., Butterworth A. E., Kimani G., Kamau T., Fulford A. J., Dunne D. W., Ouma J. H., Sturrock R. F. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993 Dec;61(12):4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. G., Shackelford P. G., Briles D. E., Nahm M. H. Human IgG subclasses and their relation to carbohydrate antigen immunocompetence. Diagn Clin Immunol. 1988;5(5):241–248. [PubMed] [Google Scholar]
- Shackelford P. G., Nelson S. J., Palma A. T., Nahm M. H. Human antibodies to group A streptococcal carbohydrate. Ontogeny, subclass restriction, and clonal diversity. J Immunol. 1988 May 1;140(9):3200–3205. [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., McIntyre T. M., Mandler R., Pecanha L. M., Finkelman F. D., Lees A., Mond J. J. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992 May 1;175(5):1367–1371. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Mond J. J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993 Jan;14(1):15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., O'Connor R. D., Falkoff R. J., Beck L. Interleukin-4 induced IgE and IgG4 secretion by B cells from atopic dermatitis patients. Int Arch Allergy Appl Immunol. 1991;94(1-4):181–183. doi: 10.1159/000235357. [DOI] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]