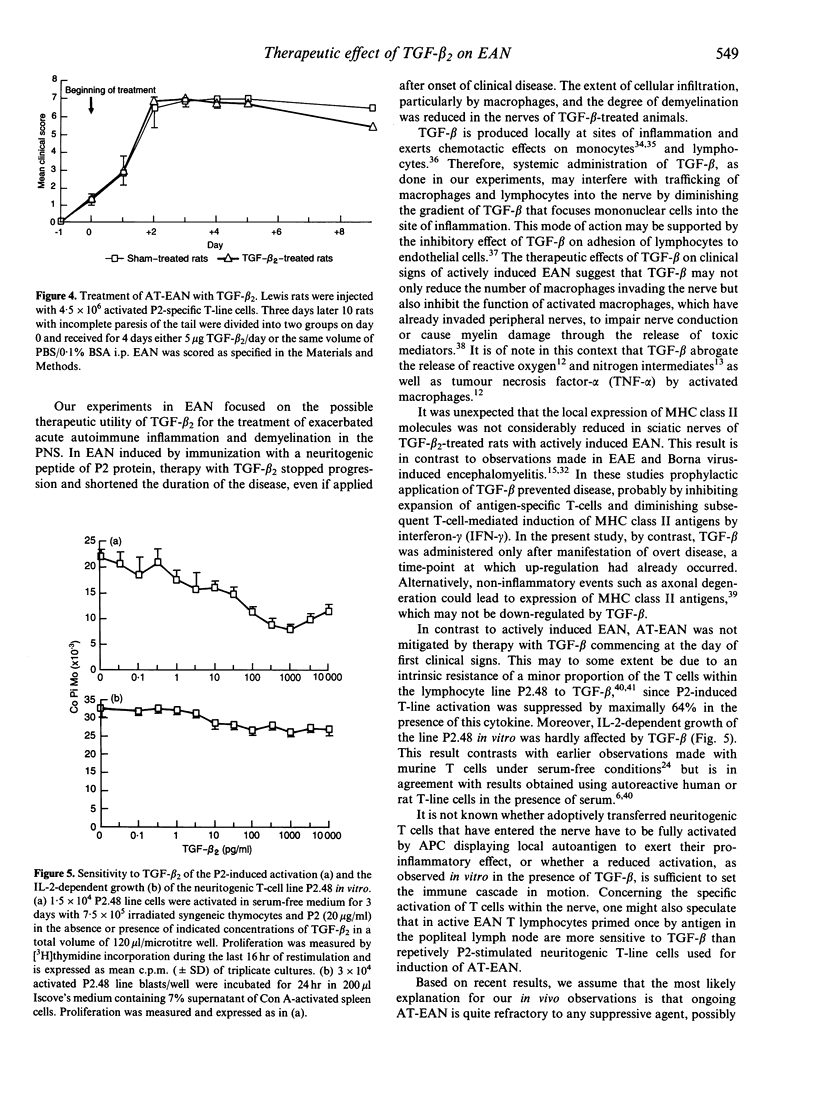
Abstract
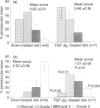
A possible effect of transforming growth factor type-beta 2 (TGF-beta 2) on autoimmune inflammation of the peripheral nervous system (PNS) was evaluated in experimental autoimmune neuritis (EAN) in Lewis rats, a disease model of the human Guillain-Barré syndrome. First, EAN was actively induced by immunization with a neuritogenic peptide corresponding to amino acids 53-78 of the bovine P2 protein. Intraperitoneal (i.p.) administration of 5 micrograms TGF-beta 2 per day after onset of clinical disease shortened the duration and ameliorated the severity of EAN compared to sham-injected control animals. Inflammatory infiltration and demyelination was significantly reduced in sciatic nerves of TGF-beta-treated animals, although expression of major histocompatibility complex (MHC) class II antigens was not down-regulated. Second, EAN was induced by adoptive transfer (AT) of activated P2-specific T-line cells (AT-EAN). Daily injections of 5 micrograms TGF-beta 2 i.p., beginning on the day of first clinical signs, failed to modify the clinical course of AT-EAN, although the antigen-induced activation of the neuritogenic T-line cells used for induction of disease was found to be partially sensitive to the inhibitory effect of TGF-beta in vitro. The experiments indicate that TGF-beta 2 holds promise as a therapeutic agent to combat autoimmunity in the PNS. They also suggest that the therapeutic efficacy of TGF-beta on rapidly developing disease such as AT-EAN is limited, as with other non-specific immunosuppressive drugs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Hathaway M., Shaw J., Burnett D., Elias E., Strain A. J. Transforming growth factor-beta induces human T lymphocyte migration in vitro. J Immunol. 1991 Jul 15;147(2):609–612. [PubMed] [Google Scholar]
- Allen J. B., Manthey C. L., Hand A. R., Ohura K., Ellingsworth L., Wahl S. M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J Exp Med. 1990 Jan 1;171(1):231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. B., Manthey C. L., Hand A. R., Ohura K., Ellingsworth L., Wahl S. M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J Exp Med. 1990 Jan 1;171(1):231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M. E., Allen J. B., Ogawa Y., Wahl S. M. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J Clin Invest. 1991 Mar;87(3):1108–1113. doi: 10.1172/JCI115073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Fontana A., Frei K., Bodmer S., Hofer E., Schreier M. H., Palladino M. A., Jr, Zinkernagel R. M. Transforming growth factor-beta inhibits the generation of cytotoxic T cells in virus-infected mice. J Immunol. 1989 Nov 15;143(10):3230–3234. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Gamble J. R., Vadas M. A. Endothelial cell adhesiveness for human T lymphocytes is inhibited by transforming growth factor-beta 1. J Immunol. 1991 Feb 15;146(4):1149–1154. [PubMed] [Google Scholar]
- Hartung H. P., Heininger K., Schäfer B., Fierz W., Toyka K. V. Immune mechanisms in inflammatory polyneuropathy. Ann N Y Acad Sci. 1988;540:122–161. doi: 10.1111/j.1749-6632.1988.tb27058.x. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Schäfer B., Diamantstein T., Fierz W., Heininger K., Toyka K. V. Suppression of P2-T cell line-mediated experimental autoimmune neuritis by interleukin-2 receptor targeted monoclonal antibody ART 18. Brain Res. 1989 Jun 5;489(1):120–128. doi: 10.1016/0006-8993(89)90014-0. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Toyka K. V. T-cell and macrophage activation in experimental autoimmune neuritis and Guillain-Barré syndrome. Ann Neurol. 1990;27 (Suppl):S57–S63. doi: 10.1002/ana.410270716. [DOI] [PubMed] [Google Scholar]
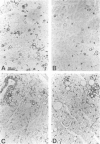
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Johns L. D., Flanders K. C., Ranges G. E., Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta 1. J Immunol. 1991 Sep 15;147(6):1792–1796. [PubMed] [Google Scholar]
- Johns L. D., Sriram S. Experimental allergic encephalomyelitis: neutralizing antibody to TGF beta 1 enhances the clinical severity of the disease. J Neuroimmunol. 1993 Aug;47(1):1–7. doi: 10.1016/0165-5728(93)90278-7. [DOI] [PubMed] [Google Scholar]
- Jung S., Krämer S., Schluesener H. J., Hünig T., Toyka K., Hartung H. P. Prevention and therapy of experimental autoimmune neuritis by an antibody against T cell receptors-alpha/beta. J Immunol. 1992 Jun 15;148(12):3768–3775. [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991 Feb 15;146(4):1163–1168. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Suzuki M., Uyemura K. Purification and partial characterization of two glycoproteins in bovine peripheral nerve myelin membrane. Biochim Biophys Acta. 1976 Dec 14;455(3):806–816. doi: 10.1016/0005-2736(76)90050-x. [DOI] [PubMed] [Google Scholar]
- Kuruvilla A. P., Shah R., Hochwald G. M., Liggitt H. D., Palladino M. A., Thorbecke G. J. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Ellingsworth L. R., Gillis S., Wall R., Kincade P. W. Beta transforming growth factors are potential regulators of B lymphopoiesis. J Exp Med. 1987 Nov 1;166(5):1290–1299. doi: 10.1084/jem.166.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linington C., Izumo S., Suzuki M., Uyemura K., Meyermann R., Wekerle H. A permanent rat T cell line that mediates experimental allergic neuritis in the Lewis rat in vivo. J Immunol. 1984 Oct;133(4):1946–1950. [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olee T., Weise M., Powers J., Brostoff S. A T cell epitope for experimental allergic neuritis is an amphipathic alpha-helical structure. J Neuroimmunol. 1989 Feb;21(2-3):235–240. doi: 10.1016/0165-5728(89)90179-3. [DOI] [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Racke M. K., Cannella B., Albert P., Sporn M., Raine C. S., McFarlin D. E. Evidence of endogenous regulatory function of transforming growth factor-beta 1 in experimental allergic encephalomyelitis. Int Immunol. 1992 May;4(5):615–620. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- Racke M. K., Dhib-Jalbut S., Cannella B., Albert P. S., Raine C. S., McFarlin D. E. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J Immunol. 1991 May 1;146(9):3012–3017. [PubMed] [Google Scholar]
- Ranges G. E., Figari I. S., Espevik T., Palladino M. A., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Kehrl J. H., Wakefield L. M., Roberts A. B., Sporn M. B., Burlington D. B., Lane H. C., Fauci A. S. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986 May 15;136(10):3916–3920. [PubMed] [Google Scholar]
- Santambrogio L., Hochwald G. M., Saxena B., Leu C. H., Martz J. E., Carlino J. A., Ruddle N. H., Palladino M. A., Gold L. I., Thorbecke G. J. Studies on the mechanisms by which transforming growth factor-beta (TGF-beta) protects against allergic encephalomyelitis. Antagonism between TGF-beta and tumor necrosis factor. J Immunol. 1993 Jul 15;151(2):1116–1127. [PubMed] [Google Scholar]
- Schluesener H. J., Lider O. Transforming growth factors beta 1 and beta 2: cytokines with identical immunosuppressive effects and a potential role in the regulation of autoimmune T cell function. J Neuroimmunol. 1989 Oct;24(3):249–258. doi: 10.1016/0165-5728(89)90123-9. [DOI] [PubMed] [Google Scholar]
- Schluesener H., Jung S., Salvetti M. Susceptibility and resistance of human autoimmune T cell activation to the immunoregulatory effects of transforming growth factor (TGF) beta 1, beta 2, and beta 1.2. J Neuroimmunol. 1990 Aug;28(3):271–276. doi: 10.1016/0165-5728(90)90020-n. [DOI] [PubMed] [Google Scholar]
- Siepl C., Bodmer S., Frei K., MacDonald H. R., De Martin R., Hofer E., Fontana A. The glioblastoma-derived T cell suppressor factor/transforming growth factor-beta 2 inhibits T cell growth without affecting the interaction of interleukin 2 with its receptor. Eur J Immunol. 1988 Apr;18(4):593–600. doi: 10.1002/eji.1830180416. [DOI] [PubMed] [Google Scholar]
- Siepl C., Malipiero U. V., Fontana A. Transforming growth factor-beta (TGF-beta)-resistant helper T lymphocyte clones show a concomitant loss of all three types of TGF-beta receptors. J Immunol. 1991 May 1;146(9):3063–3067. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz L., Planz O., Bilzer T., Frei K., Fontana A. Transforming growth factor-beta modulates T cell-mediated encephalitis caused by Borna disease virus. Pathogenic importance of CD8+ cells and suppression of antibody formation. J Immunol. 1991 Nov 15;147(10):3581–3586. [PubMed] [Google Scholar]
- Stoll G., Griffin J. W., Li C. Y., Trapp B. D. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989 Oct;18(5):671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Mergenhagen S. E. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989 Aug;10(8):258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Winokur T. S., Hollands R. S., Christopherson K., Levinson A. D., Sporn M. B. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990 Dec;86(6):1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]