Abstract
Dendritic cells (DC) are the most potent antigen-presenting cells (APC) involved in the initiation of primary T-lymphocyte responses. However, despite their importance, no DC-specific surface marker has been identified in humans and many aspects of their ontogeny and the mechanisms underlying their potent functional activity remain unknown. In this report we describe a novel monoclonal antibody (mAb), CMRF-44, which recognizes an early activation antigen expressed by human DC and acts as a marker of allostimulatory activity within preparations of peripheral blood mononuclear cells (PBMC). The CMRF-44 antigen was expressed strongly on DC isolated from blood and tonsil by standard techniques, but was not detectable on Langerhans' cells within skin or on DC isolated directly from blood using a cell-sorting method which involves minimal DC manipulation/activation. Normal resting peripheral blood leucocytes did not label with CMRF-44, although weak staining of a small subpopulation (15%) of blood B lymphocytes was identified by double labelling. However, following overnight culture at 37 degrees, moderate staining of a subpopulation of PBMC was detected. Confirmation that CMRF-44 recognized an early marker of activated DC and hence allostimulatory activity was obtained by sorting cultured cell preparations on the basis of CMRF-44 reactivity. A marked enrichment of allostimulatory activity was observed in the CMRF-44-positive cellular population, whereas the CMRF-44-negative cells showed only minimal stimulatory activity. Activation studies established that the CMRF-44 antigen was an early activation marker, expressed constitutively on the majority of tonsil B lymphocytes, which can be induced on peripheral blood B lymphocytes and subpopulations of monocytes. Expression of the CMRF-44 antigen on cell lines was similarly restricted, CMRF-44 antigen being detected only on Hodgkin's disease-derived and B-lymphoid lines. The cellular distribution, expression kinetics and biochemical characteristics of the CMRF-44 antigen identify it as a new early marker of activated allostimulatory (DC) populations.
Full text
PDF



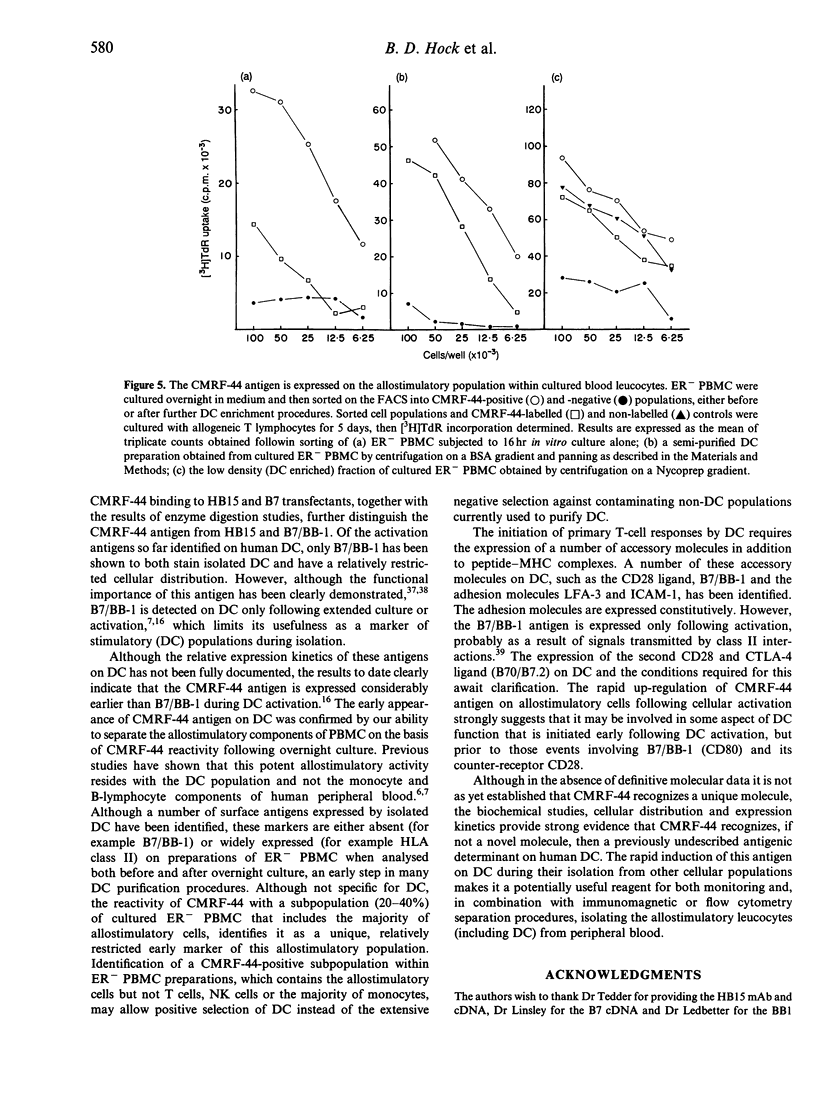

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Cole S. R., Ashman L. K., Ey P. L. Biotinylation: an alternative to radioiodination for the identification of cell surface antigens in immunoprecipitates. Mol Immunol. 1987 Jul;24(7):699–705. doi: 10.1016/0161-5890(87)90051-4. [DOI] [PubMed] [Google Scholar]
- Crow M. K., Kunkel H. G. Human dendritic cells: major stimulators of the autologous and allogeneic mixed leucocyte reactions. Clin Exp Immunol. 1982 Aug;49(2):338–346. [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- Freedman A. S., Boyd A. W., Anderson K. C., Fisher D. C., Schlossman S. F., Nadler L. M. B5, a new B cell-restricted activation antigen. J Immunol. 1985 Apr;134(4):2228–2235. [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. N., McKenzie J. L. Interstitial dendritic cells. Int Rev Immunol. 1990;6(2-3):127–138. doi: 10.3109/08830189009056624. [DOI] [PubMed] [Google Scholar]
- Hart D. N., McKenzie J. L. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988 Jul 1;168(1):157–170. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. N., Prickett T. C. Intercellular adhesion molecule-2 (ICAM-2) expression on human dendritic cells. Cell Immunol. 1993 May;148(2):447–454. doi: 10.1006/cimm.1993.1126. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Starling G. C., Calder V. L., Fernando N. S. B7/BB-1 is a leucocyte differentiation antigen on human dendritic cells induced by activation. Immunology. 1993 Aug;79(4):616–620. [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hsu P. L., Hsu S. M. Identification of an Mr 70,000 antigen associated with Reed-Sternberg cells and interdigitating reticulum cells. Cancer Res. 1990 Jan 15;50(2):350–357. [PubMed] [Google Scholar]
- Hsu S. M. The never-ending controversies in Hodgkin's disease. Blood. 1990 Apr 15;75(8):1742–1744. [PubMed] [Google Scholar]
- Inaba K., Witmer M. D., Steinman R. M. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells during primary antibody responses in vitro. J Exp Med. 1984 Sep 1;160(3):858–876. doi: 10.1084/jem.160.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung L. K., Fu S. M. Selective inhibition of growth factor-dependent human B cell proliferation by monoclonal antibody AB1 to an antigen expressed by activated B cells. J Exp Med. 1984 Dec 1;160(6):1919–1924. doi: 10.1084/jem.160.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E. Possible origin of the Reed-Sternberg cell from an interdigitating reticulum cell. Cancer Treat Rep. 1982 Apr;66(4):601–608. [PubMed] [Google Scholar]
- Koulova L., Clark E. A., Shu G., Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991 Mar 1;173(3):759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince C., Richard Y., Krief P., Treton D., Boucheix C., Galanaud P. A B cell-restricted activation antigen (B8.7) functionally related to the low molecular weight B cell growth factor receptor. J Immunol. 1988 Jan 1;140(1):100–107. [PubMed] [Google Scholar]
- MacPherson G. G. What don't we know about dendritic cells? Res Immunol. 1989 Nov-Dec;140(9):877–926. doi: 10.1016/0923-2494(89)90046-1. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V., Blaszczyk M., Mitchell K. F., Steplewski Z., Koprowski H. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981 Apr 3;212(4490):55–56. doi: 10.1126/science.7209516. [DOI] [PubMed] [Google Scholar]
- McKenzie J. L., Egner W., Calder V. L., Hart D. N. Hodgkin's disease cell lines: a model for interleukin-1-independent accessory cell function. Immunology. 1992 Nov;77(3):345–353. [PMC free article] [PubMed] [Google Scholar]
- Nabavi N., Freeman G. J., Gault A., Godfrey D., Nadler L. M., Glimcher L. H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992 Nov 19;360(6401):266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Prickett T. C., McKenzie J. L., Hart D. N. Adhesion molecules on human tonsil dendritic cells. Transplantation. 1992 Feb;53(2):483–490. doi: 10.1097/00007890-199202010-00041. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Maino V. C., Warner N. L., Brodsky F. M. Activated T cells and monocytes have characteristic patterns of class II antigen expression. J Immunol. 1988 Aug 15;141(4):1281–1287. [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sanders S. K., Butler J. L., Gartland G. L., Komiyama K., Cooper M. D. Identification of an early activation antigen (Bac-1) on human B cells. J Immunol. 1986 Aug 15;137(4):1208–1213. [PubMed] [Google Scholar]
- Tedder T. F., McIntyre G., Schlossman S. F. Heterogeneity in the B1 (CD20) cell surface molecule expressed by human B-lymphocytes. Mol Immunol. 1988 Dec;25(12):1321–1330. doi: 10.1016/0161-5890(88)90047-8. [DOI] [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Nadler L. M., Bhan A. K., Schooley R. T. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. J Immunol. 1985 May;134(5):3007–3012. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Schooley R. T., Bhan A. K., Nadler L. M. Epstein-Barr virus superinduces a new human B cell differentiation antigen (B-LAST 1) expressed on transformed lymphoblasts. Cell. 1982 Sep;30(2):415–425. doi: 10.1016/0092-8674(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]
- Young J. W., Koulova L., Soergel S. A., Clark E. A., Steinman R. M., Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992 Jul;90(1):229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. J., Schwarting R., Smith H. M., Tedder T. F. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992 Jul 15;149(2):735–742. [PubMed] [Google Scholar]