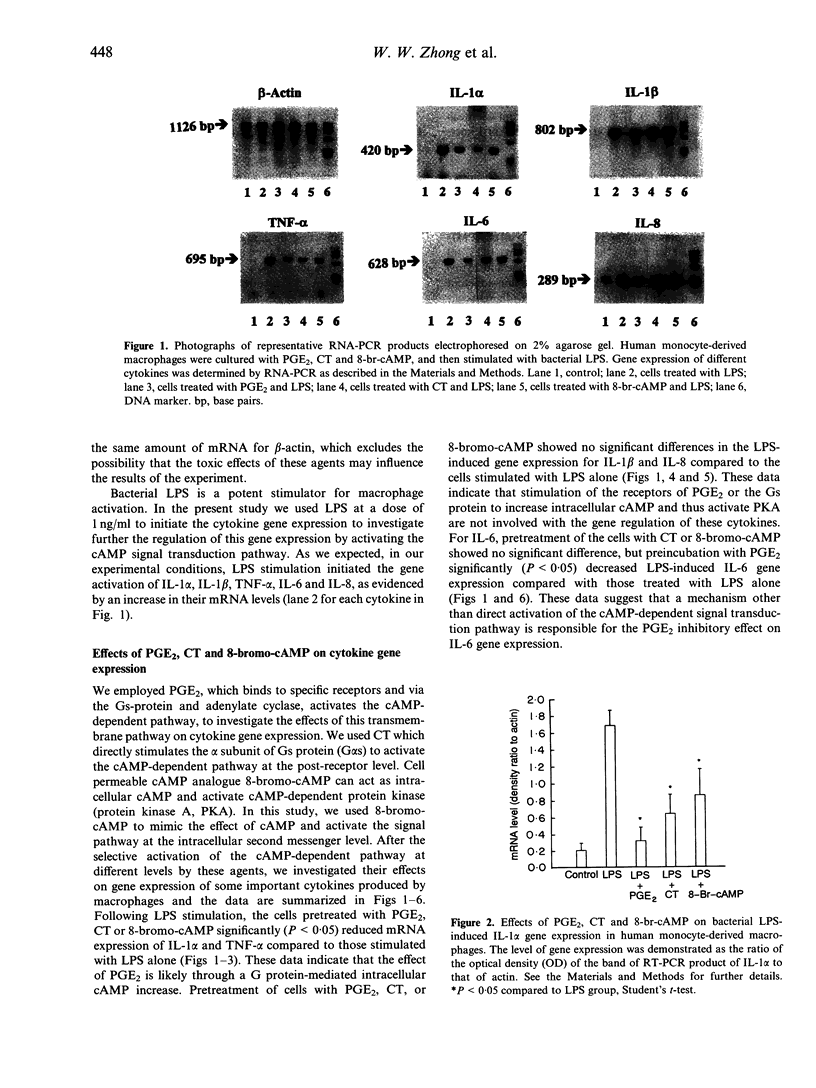
Abstract
Prostaglandin E2 (PGE2) appears to regulate macrophage cytokine production through the stimulatory GTP-binding protein (Gs protein)-mediated cyclic AMP (cAMP)-dependent transmembrane signal transduction pathway. In this study, we used PGE2, cholera toxin (CT; a direct G alpha s protein stimulator) and 8-bromo-cAMP (a membrane permeable cAMP analogue) to stimulate this pathway, and investigated their influence on cytokine gene expression in lipopolysaccharide (LPS)-activated human macrophages. The mRNA expression for interleukin-1 alpha (IL-1 alpha), IL-1 beta, tumour necrosis factor-alpha (TNF-alpha), IL-6 and IL-8 were determined employing reverse transcription polymerase chain reaction (RT-PCR) using specific primers. We demonstrated that PGE2, CT and 8-bromo-cAMP inhibited the LPS-induced gene activation of TNF-alpha and IL-1 alpha, and had no effect on the gene activation of IL-1 beta and IL-8. Further, our data indicate that PGE2 suppressed the gene activation of IL-6 following LPS stimulation, but neither CT nor 8-bromo-cAMP had an effect. These data suggest that PGE2 alters LPS-stimulated gene activation of only some of the early macrophage cytokines, and does so either by a Gs transmembrane cAMP-dependent or an independent system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly S., Ferrua B., Fay M., Gougerot-Pocidalo M. A. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990 May;2(3):205–210. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Figueíredo F., Uhing R. J., Okonogi K., Gettys T. W., Johnson S. P., Adams D. O., Prpic V. Activation of the cAMP cascade inhibits an early event involved in murine macrophage Ia expression. J Biol Chem. 1990 Jul 25;265(21):12317–12323. [PubMed] [Google Scholar]
- Funaki N., Arii S., Adachi Y., Higashituji H., Fujita S., Furutani M., Mise M., Ishiguro S., Kitao T., Tanaka J. Effect of PGE2 on interleukin-1 and superoxide release from primary-cultured human hepatic macrophages. Life Sci. 1992;51(17):1339–1346. doi: 10.1016/0024-3205(92)90633-z. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Hurme M. Modulation of interleukin-1 beta production by cyclic AMP in human monocytes. FEBS Lett. 1990 Apr 9;263(1):35–37. doi: 10.1016/0014-5793(90)80699-j. [DOI] [PubMed] [Google Scholar]
- Hurme M., Serkkola E., Ronni T., Silvennoinen O. Control of interleukin-1 beta expression by protein kinase C and cyclic adenosine monophosphate in myeloid leukemia cells. Blood. 1990 Dec 1;76(11):2198–2203. [PubMed] [Google Scholar]
- Iwaz J., Kouassi E., Lafont S., Revillard J. P. Elevation of cyclic adenosine monophosphate levels independently down regulates IL-1, IL-2, and IL-2 receptor (CD25) syntheses. Int J Immunopharmacol. 1990;12(6):631–637. doi: 10.1016/0192-0561(90)90100-2. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Konopski Z., Seljelid R., Eskeland T. Cytokines and PGE2 modulate the phagocytic function of the beta-glucan receptor in macrophages. Scand J Immunol. 1993 May;37(5):587–592. doi: 10.1111/j.1365-3083.1993.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Parhar R. S., Singh P. Indomethacin therapy abrogates the prostaglandin-mediated suppression of natural killer activity in tumor-bearing mice and prevents tumor metastasis. Cell Immunol. 1986 Apr 15;99(1):108–118. doi: 10.1016/0008-8749(86)90220-0. [DOI] [PubMed] [Google Scholar]
- Leung K. H. Inhibition of human NK cell and LAK cell cytotoxicity and differentiation by PGE2. Cell Immunol. 1989 Oct 15;123(2):384–395. doi: 10.1016/0008-8749(89)90298-0. [DOI] [PubMed] [Google Scholar]
- Mattana J., Singhal P. C. Effects of prostaglandin E2 and cyclic AMP on uptake of immunoglobulin G complexes by cultured macrophages. Eicosanoids. 1992;5(2):67–72. [PubMed] [Google Scholar]
- Ohmori Y., Strassman G., Hamilton T. A. cAMP differentially regulates expression of mRNA encoding IL-1 alpha and IL-1 beta in murine peritoneal macrophages. J Immunol. 1990 Nov 15;145(10):3333–3339. [PubMed] [Google Scholar]
- Parhar R. S., Lala P. K. Changes in the host natural killer cell population in mice during tumor development. 2. The mechanism of suppression of NK activity. Cell Immunol. 1985 Jul;93(2):265–279. doi: 10.1016/0008-8749(85)90133-9. [DOI] [PubMed] [Google Scholar]
- Phipps R. P., Roper R. L., Stein S. H. Regulation of B-cell tolerance and triggering by macrophages and lymphoid dendritic cells. Immunol Rev. 1990 Oct;117:135–158. doi: 10.1111/j.1600-065x.1990.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Phipps R. P., Stein S. H., Roper R. L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991 Oct;12(10):349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- Prpic V., Weiel J. E., Somers S. D., DiGuiseppi J., Gonias S. L., Pizzo S. V., Hamilton T. A., Herman B., Adams D. O. Effects of bacterial lipopolysaccharide on the hydrolysis of phosphatidylinositol-4,5-bisphosphate in murine peritoneal macrophages. J Immunol. 1987 Jul 15;139(2):526–533. [PubMed] [Google Scholar]
- Rao C. V., Mitra S. B. Distribution of PGE and PGF2 alpha receptor proteins in the intracellular organelles of bovine corpora lutea. Methods Enzymol. 1982;86:192–202. doi: 10.1016/0076-6879(82)86190-9. [DOI] [PubMed] [Google Scholar]
- Santarpia G., Valenti A., Loteta L., Sofo V., Sottile A., Serrao N., Salmeri F. M., Teti D. A short incubation with PGE2 affects the proliferative response of T lymphocytes to PWM. Immunopharmacol Immunotoxicol. 1992;14(4):757–767. doi: 10.3109/08923979209009233. [DOI] [PubMed] [Google Scholar]
- Schad V. C., Phipps R. P. Two signals are required for accessory cells to induce B cell unresponsiveness. Tolerogenic Ig and prostaglandin. J Immunol. 1988 Jul 1;141(1):79–84. [PubMed] [Google Scholar]
- Serkkola E., Hurme M., Palkama T. Prolonged elevation of intracellular cyclic AMP activates interleukin-1 production in human peripheral blood monocytes. Scand J Immunol. 1992 Feb;35(2):203–208. doi: 10.1111/j.1365-3083.1992.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Shimozato T., Iwata M., Kawada H., Tamura N. Human immunoglobulin preparation for intravenous use induces elevation of cellular cyclic adenosine 3':5'-monophosphate levels, resulting in suppression of tumour necrosis factor alpha and interleukin-1 production. Immunology. 1991 Apr;72(4):497–501. [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Skinner S., Marbrook J. The effect of prostaglandins and indomethacin on cytotoxic T-lymphocytes and their precursors. Int J Immunopharmacol. 1989;11(3):267–273. doi: 10.1016/0192-0561(89)90164-1. [DOI] [PubMed] [Google Scholar]
- Spengler R. N., Spengler M. L., Lincoln P., Remick D. G., Strieter R. M., Kunkel S. L. Dynamics of dibutyryl cyclic AMP- and prostaglandin E2-mediated suppression of lipopolysaccharide-induced tumor necrosis factor alpha gene expression. Infect Immun. 1989 Sep;57(9):2837–2841. doi: 10.1128/iai.57.9.2837-2841.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Rolfe M. W., Evanoff H. L., Allen R. M., Strieter R. M. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol. 1992 Jan;6(1):75–81. doi: 10.1165/ajrcmb/6.1.75. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Walters J. A. Increased cyclic AMP levels enhance IL-1 alpha and IL-1 beta mRNA expression and protein production in human myelomonocytic cell lines and monocytes. J Clin Invest. 1991 Dec;88(6):1915–1923. doi: 10.1172/JCI115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilden A. B., Balch C. M. Indomethacin enhancement of immunocompetence in melanoma patients. Surgery. 1981 Jul;90(1):77–84. [PubMed] [Google Scholar]
- Valitutti S., Castellino F., Aiello F. B., Ricci R., Patrignani P., Musiani P. The role of arachidonic acid metabolite PGE2 on T cell proliferative response. J Clin Lab Immunol. 1989 Aug;29(4):167–173. [PubMed] [Google Scholar]
- Viherluoto J., Palkama T., Silvennoinen O., Hurme M. Cyclic adenosine monophosphate decreases the secretion, but not the cell-associated levels, of interleukin-1 beta in lipopolysaccharide-activated human monocytes. Scand J Immunol. 1991 Jul;34(1):121–125. doi: 10.1111/j.1365-3083.1991.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- Waymack J. P., Guzman R. F., Burleson D. G., McManus A. T., Mason A. D., Pruitt B. A., Jr Effect of prostaglandin E in multiple experimental models. VI. Effect on T-cell subsets. Prostaglandins. 1989 Sep;38(3):345–353. doi: 10.1016/0090-6980(89)90138-x. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Suzuki T. Prostaglandin-E2-induced activation of adenosine 3'-5' cyclic monophosphate-dependent protein kinases of a murine macrophage-like cell line (P388D1). J Immunol. 1987 Nov 15;139(10):3416–3421. [PubMed] [Google Scholar]
- Zhong W. W., Burke P. A., Hand A. T., Walsh M. J., Hughes L. A., Forse R. A. Regulation of cytokine mRNA expression in lipopolysaccharide-stimulated human macrophages. Arch Surg. 1993 Feb;128(2):158–164. doi: 10.1001/archsurg.1993.01420140035006. [DOI] [PubMed] [Google Scholar]