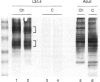
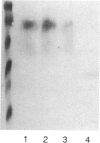
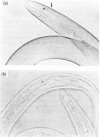
Abstract
The ability to identify antigens associated with an infection has generally relied on the use of serum antibodies produced by infected or previously exposed individuals. A major drawback with the use of serum is that it does not necessarily reflect the local antibody response at mucosal tissue sites. This study describes an approach that allows the use of antibodies generated close to the infection site to detect the transient expression of stage-specific antigens during infection with the gastrointestinal parasite Haemonchus contortus. This was achieved by infecting immune sheep with H. contortus larvae and removing the abomasal lymph nodes draining the infection site shortly after the challenge infection. Antibody-secreting cell (ASC) probes were generated from these lymph nodes after short-term in vitro culture of cell suspensions, which allowed the accumulation of antibodies secreted by in vivo-induced ASC into the culture supernatant. Lymph node culture supernatants (= ASC probes) from immune sheep challenged 5 days previously were used to probe Western blots of third and fourth stage larval preparations, and revealed distinct reactivity to larval antigens. No antibody reactivity to larval antigen preparations was detected in sheep that were not challenged. The number of antigens identified using ASC probes was significantly restricted compared to either pre- or post-challenge sera. In contrast to the variability of the serum response, the specificity of ASC probes was highly repeatable between different sheep. ASC probes were also used to purify a H. contortus larval antigen by affinity chromatography, which allowed limited biochemical studies to be undertaken. The antigen(s) recognized by the ASC probes were shown to be expressed on the surface of the larvae. These studies illustrate the use of a novel means of studying the local antibody response close to a mucosal infection site in order to identify and isolate stage-specific antigens expressed during infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anya A. O. The structure and chemical composition of the nematode cuticle. Observations on some oxyurids and Ascaris. Parasitology. 1966 Feb;56(1):179–198. doi: 10.1017/s0031182000071201. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cox G. N., Shamansky L. M., Boisvenue R. J. Identification and preliminary characterization of cuticular surface proteins of Haemonchus contortus. Mol Biochem Parasitol. 1989 Oct;36(3):233–241. doi: 10.1016/0166-6851(89)90171-0. [DOI] [PubMed] [Google Scholar]
- Grencis R. K., Crawford C., Pritchard D. I., Behnke J. M., Wakelin D. Immunization of mice with surface antigens from the muscle larvae of Trichinella spiralis. Parasite Immunol. 1986 Nov;8(6):587–596. doi: 10.1111/j.1365-3024.1986.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Kazura J. W., Grove D. I. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis. Nature. 1978 Aug 10;274(5671):588–589. doi: 10.1038/274588a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maizels R. M., Kennedy M. W., Meghji M., Robertson B. D., Smith H. V. Shared carbohydrate epitopes on distinct surface and secreted antigens of the parasitic nematode Toxocara canis. J Immunol. 1987 Jul 1;139(1):207–214. [PubMed] [Google Scholar]
- Meeusen E. N., Brandon M. R. Antibody secreting cells as specific probes for antigen identification. J Immunol Methods. 1994 Jun 3;172(1):71–76. doi: 10.1016/0022-1759(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Meeusen E., Barcham G. J., Gorrell M. D., Rickard M. D., Brandon M. R. Cysticercosis: cellular immune responses during primary and secondary infection. Parasite Immunol. 1990 Jul;12(4):403–418. doi: 10.1111/j.1365-3024.1990.tb00977.x. [DOI] [PubMed] [Google Scholar]
- Meeusen E., Brandon M. The use of antibody-secreting cell probes to reveal tissue-restricted immune responses during infection. Eur J Immunol. 1994 Feb;24(2):469–474. doi: 10.1002/eji.1830240231. [DOI] [PubMed] [Google Scholar]
- Meeusen E., Lee C. S., Brandon M. Differential migration of T and B cells during an acute inflammatory response. Eur J Immunol. 1991 Sep;21(9):2269–2272. doi: 10.1002/eji.1830210940. [DOI] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnka Z., Cahill R. N. Aspects of the immune response in single lymph nodes. Monogr Allergy. 1980;16:245–259. [PubMed] [Google Scholar]
- Turnbull I. F., Bowles V. M., Wiltshire C. J., Brandon M. R., Meeusen E. N. Systemic immunization of sheep with surface antigens from Haemonchus contortus larvae. Int J Parasitol. 1992 Jul;22(4):537–540. doi: 10.1016/0020-7519(92)90157-g. [DOI] [PubMed] [Google Scholar]
- Wilson R. A., Coulson P. S. Lung-phase immunity to Schistosomes: a new perspective on an old problem? Parasitol Today. 1989 Sep;5(9):274–278. doi: 10.1016/0169-4758(89)90017-3. [DOI] [PubMed] [Google Scholar]