Abstract
Human membrane cofactor protein (MCP, CD46) functions as an inhibitor of the complement (C) cascade to protect host cells from C attack, and as a receptor for measles virus (MV). Normal human sera contains 10-60 ng/ml of naturally produced soluble forms of MCP, which is also a cofactor for the factor I-mediated inactivation of C3b. We produced monoclonal antibodies (mAb) against MCP and a recombinant soluble form of MCP similar to the natural soluble forms, and tested their ability to block MV infection. Vero cells and CHO cells expressing human MCP were the targets. Of the antibodies tested, M75 and M177, which blocked the C regulatory activity of MCP, efficiently blocked MV infection. More than 50 micrograms/ml of the soluble form moderately blocked MV infection of CHO cells expressing MCP, but barely blocked that of Vero cells. The two mAb and the soluble form also inhibited MV H protein-mediated green monkey erythrocyte rosette formation. A quantitative analysis suggested that 30 micrograms/ml of the soluble form functionally corresponded to 0.2 microgram/ml of M177 or M75. These data established that the C regulatory function and the MV receptor function of MCP were blocked simultaneously by the individual mAb, and that soluble forms of MCP could inhibit MV infection in cells expressing human MCP, although doses far higher than the natural concentration of soluble MCP were required.
Full text
PDF


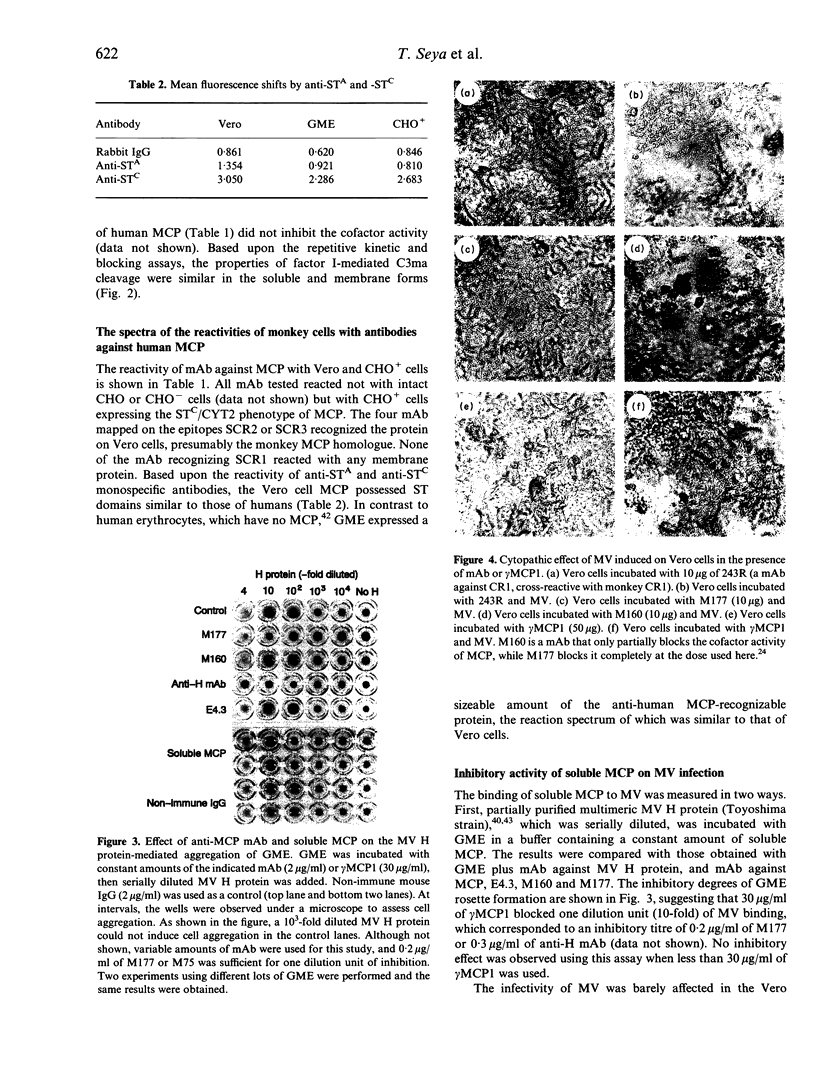



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. M., Brown M. C., Nunge M., Krych M., Atkinson J. P. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991 Nov 1;147(9):3005–3011. [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R. Complement evasion strategies of microorganisms. Immunol Today. 1991 Sep;12(9):327–331. doi: 10.1016/0167-5699(91)90010-Q. [DOI] [PubMed] [Google Scholar]
- Dierich M. P., Ebenbichler C. F., Marschang P., Füst G., Thielens N. M., Arlaud G. J. HIV and human complement: mechanisms of interaction and biological implication. Immunol Today. 1993 Sep;14(9):435–440. doi: 10.1016/0167-5699(93)90246-H. [DOI] [PubMed] [Google Scholar]
- Dunster L. M., Schneider-Schaulies J., Löffler S., Lankes W., Schwartz-Albiez R., Lottspeich F., ter Meulen V. Moesin: a cell membrane protein linked with susceptibility to measles virus infection. Virology. 1994 Jan;198(1):265–274. doi: 10.1006/viro.1994.1029. [DOI] [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- FUNAHASHI S., KITAWAKI T. STUDIES ON MEASLES VIRUS HEMAGGLUTINATION. Biken J. 1963 Jul;6:73–96. [PubMed] [Google Scholar]
- Hara T., Kojima A., Fukuda H., Masaoka T., Fukumori Y., Matsumoto M., Seya T. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br J Haematol. 1992 Oct;82(2):368–373. doi: 10.1111/j.1365-2141.1992.tb06431.x. [DOI] [PubMed] [Google Scholar]
- Hara T., Kuriyama S., Kiyohara H., Nagase Y., Matsumoto M., Seya T. Soluble forms of membrane cofactor protein (CD46, MCP) are present in plasma, tears, and seminal fluid in normal subjects. Clin Exp Immunol. 1992 Sep;89(3):490–494. doi: 10.1111/j.1365-2249.1992.tb06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Mitomo K., Fujita T., Tamura N. Characterization of three monoclonal antibodies against C3 with selective specificities. Immunology. 1987 Nov;62(3):413–417. [PMC free article] [PubMed] [Google Scholar]
- Iwai K., Fukuoka S., Fushiki T., Kido K., Sengoku Y., Semba T. Preparation of a verifiable peptide-protein immunogen: direction-controlled conjugation of a synthetic fragment of the monitor peptide with myoglobin and application for sequence analysis. Anal Biochem. 1988 Jun;171(2):277–282. doi: 10.1016/0003-2697(88)90486-1. [DOI] [PubMed] [Google Scholar]
- Iwata K., Seya T., Ariga H., Nagasawa S. Expression of a hybrid complement regulatory protein, membrane cofactor protein decay accelerating factor on Chinese hamster ovary. Comparison of its regulatory effect with those of decay accelerating factor and membrane cofactor protein. J Immunol. 1994 Apr 1;152(7):3436–3444. [PubMed] [Google Scholar]
- Johnstone R. W., Russell S. M., Loveland B. E., McKenzie I. F. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993 Oct;30(14):1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- KITAWAKI T., FUNAHAS, TOYOSHIMAKHI S. PURIFICATION AND SOME ANTIGENIC PROPERTIES OF MEASLES VIRUS HEMAGGLUTININ. Biken J. 1964 Jan;6:253–270. [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A., Iwata K., Seya T., Matsumoto M., Ariga H., Atkinson J. P., Nagasawa S. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol. 1993 Aug 1;151(3):1519–1527. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Post T. W., Atkinson J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Coyne K. E. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J Exp Med. 1991 Jul 1;174(1):35–44. doi: 10.1084/jem.174.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin D. M., Liszewski M. K., Post T. W., Arce M. A., Le Beau M. M., Rebentisch M. B., Lemons L. S., Seya T., Atkinson J. P. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988 Jul 1;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Seya T., Nagasawa S. Polymorphism and proteolytic fragments of granulocyte membrane cofactor protein (MCP, CD46) of complement. Biochem J. 1992 Jan 15;281(Pt 2):493–499. doi: 10.1042/bj2810493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby T. J., Allen C. J., Liszewski M. K., White D. J., Atkinson J. P. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med. 1992 Jun 1;175(6):1547–1551. doi: 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M., Nagira M., Kawai Y., Matzno S., Mimura T., Mayumi T. A human sperm antigen possibly involved in binding and/or fusion with zona-free hamster eggs. Fertil Steril. 1990 Dec;54(6):1121–1126. doi: 10.1016/s0015-0282(16)54015-1. [DOI] [PubMed] [Google Scholar]
- Pesando J. M., Hoffman P., Abed M. Antibody-induced antigenic modulation is antigen dependent: characterization of 22 proteins on a malignant human B cell line. J Immunol. 1986 Dec 1;137(11):3689–3695. [PubMed] [Google Scholar]
- Post T. W., Liszewski M. K., Adams E. M., Tedja I., Miller E. A., Atkinson J. P. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991 Jul 1;174(1):93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Sparrow R. L., McKenzie I. F., Purcell D. F. Tissue-specific and allelic expression of the complement regulator CD46 is controlled by alternative splicing. Eur J Immunol. 1992 Jun;22(6):1513–1518. doi: 10.1002/eji.1830220625. [DOI] [PubMed] [Google Scholar]
- Seya T., Atkinson J. P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989 Dec 1;264(2):581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Ballard L. L., Bora N. S., Kumar V., Cui W., Atkinson J. P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988 Aug;18(8):1289–1294. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990 Jul 1;145(1):238–245. [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Kiyohara H., Nakanishi I., Kinouchi T., Okabe M., Shimizu A., Akedo H. Membrane cofactor protein (MCP, CD46) in seminal plasma and on spermatozoa in normal and "sterile" subjects. Eur J Immunol. 1993 Jun;23(6):1322–1327. doi: 10.1002/eji.1830230620. [DOI] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Sugita Y., Akedo H. Complement-mediated tumor cell damage induced by antibodies against membrane cofactor protein (MCP, CD46). J Exp Med. 1990 Dec 1;172(6):1673–1680. doi: 10.1084/jem.172.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Holers V. M., Atkinson J. P. Purification and functional analysis of the polymorphic variants of the C3b/C4b receptor (CR1) and comparison with H, C4b-binding protein (C4bp), and decay accelerating factor (DAF). J Immunol. 1985 Oct;135(4):2661–2667. [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B., Schreiber R. D. Antibody-independent activation of the alternative complement pathway by measles virus-infected cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):559–562. doi: 10.1073/pnas.77.1.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Takahashi M., Kurimura T., Minekawa Y., Suzuki N. Development of extremely attenuated live measles virus vaccine (CAM-EX). Biken J. 1972 Sep;15(3):173–177. [PubMed] [Google Scholar]
- Wild T. F., Malvoisin E., Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991 Feb;72(Pt 2):439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Ueda S., Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991 May;65(5):2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y., Cubitt B. A., Oldstone M. B. Measles virus inhibits mitogen-induced T cell proliferation but does not directly perturb the T cell activation process inside the cell. Virology. 1992 Mar;187(1):280–289. doi: 10.1016/0042-6822(92)90316-h. [DOI] [PubMed] [Google Scholar]




