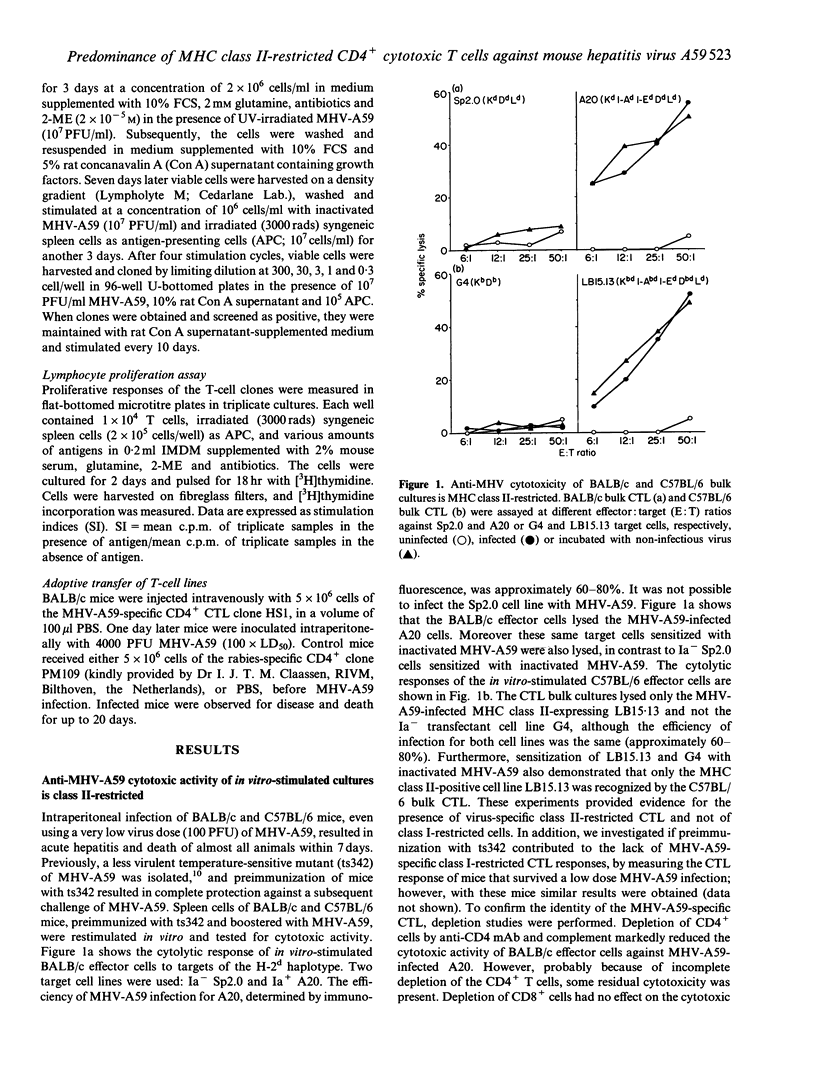
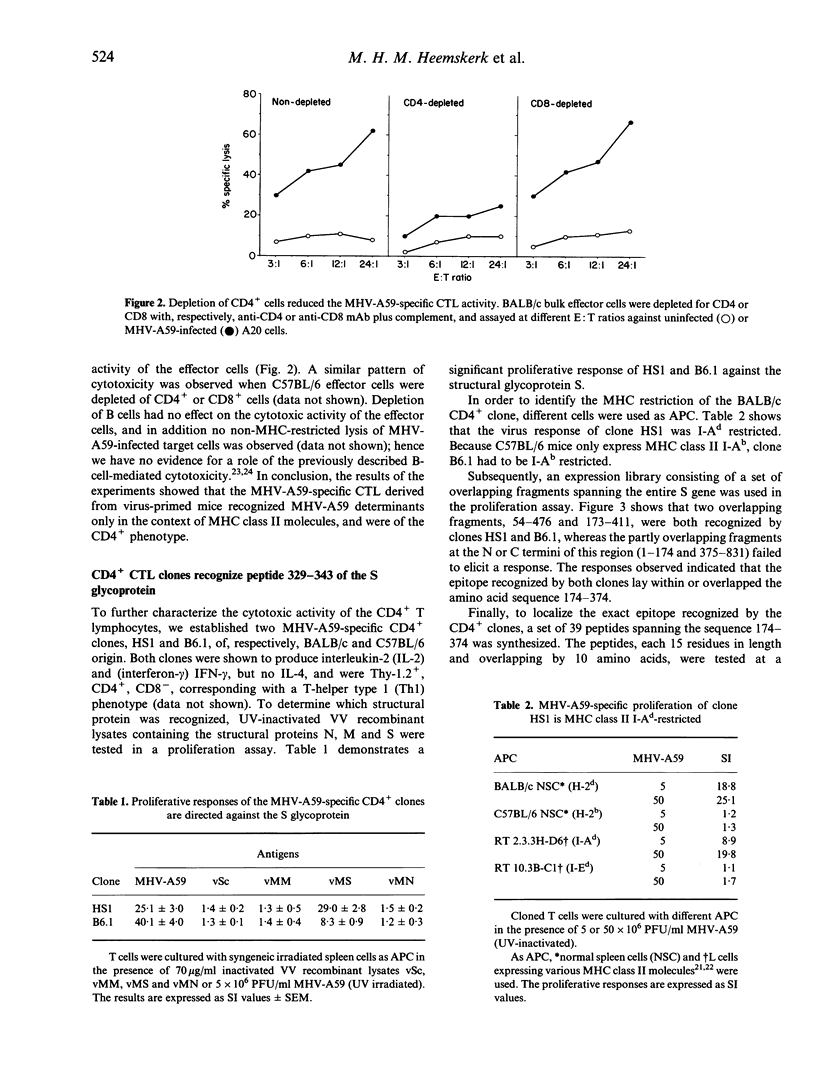
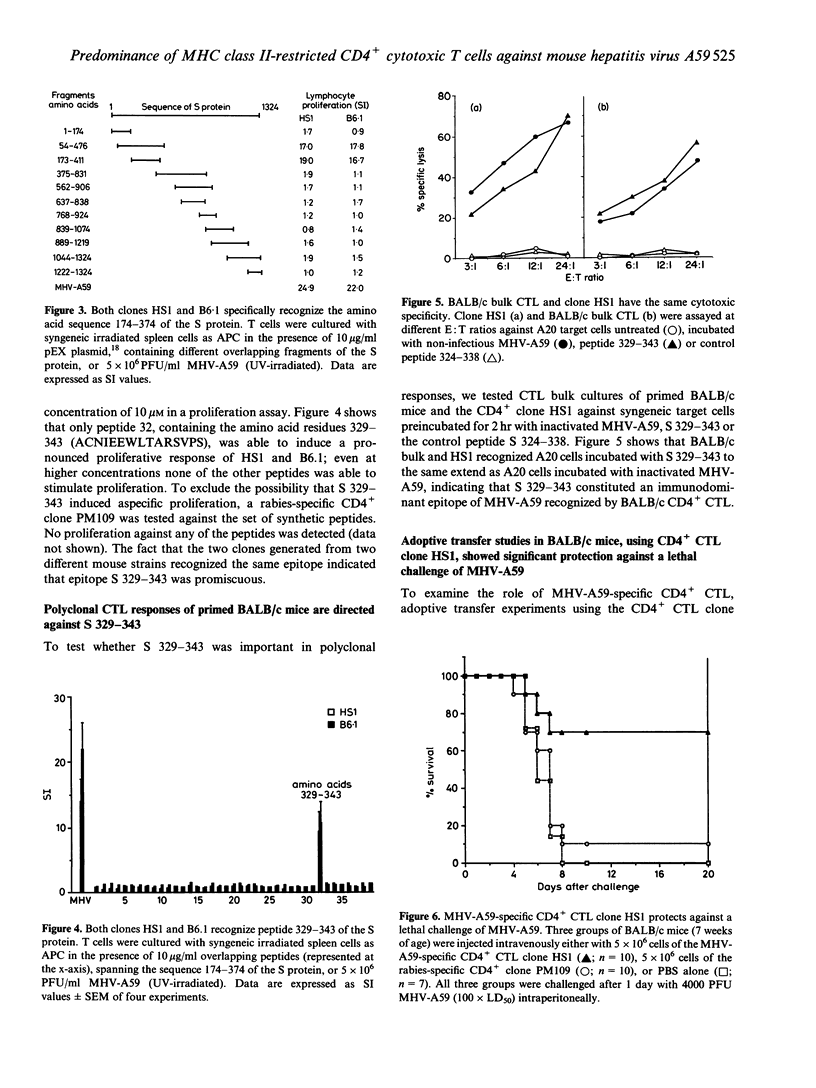
Abstract
Coronavirus-induced acute hepatitis is a complex event and the role of different components of the immune system with regard to defined viral proteins and the course of the infection is not yet clear. We have analysed the cytotoxic T-lymphocyte (CTL) response in mouse hepatitis virus (MHV-A59) infection. Surprisingly, we detected only a very clear virus-specific major histocompatibility complex (MHC) class II-restricted cytotoxicity in mice infected with MHV-A59. We found no evidence of activation of the classical CD8+ MHC class I-restricted CTL. The virus-specific CD4+ CTL derived from two different mouse strains having different MHC haplotypes recognized the same immunodominant epitope. This epitope, comprising the amino acid residues 329-343 of the viral S-glycoprotein, was recognized both at the polyclonal level and by virus-specific CTL clones. Transfer studies using a MHV-A59-specific CD4+ CTL clone showed significant protection against a lethal challenge with MHV-A59, implicating that these CD4+ CTL play a pivotal role in the protection against MHV-A59 infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Askonas B. A., Taylor P. M., Esquivel F. Cytotoxic T cells in influenza infection. Ann N Y Acad Sci. 1988;532:230–237. doi: 10.1111/j.1749-6632.1988.tb36342.x. [DOI] [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Barnes P. D., Grundy J. E. Down-regulation of the class I HLA heterodimer and beta 2-microglobulin on the surface of cells infected with cytomegalovirus. J Gen Virol. 1992 Sep;73(Pt 9):2395–2403. doi: 10.1099/0022-1317-73-9-2395. [DOI] [PubMed] [Google Scholar]
- Bergmann C., McMillan M., Stohlman S. Characterization of the Ld-restricted cytotoxic T-lymphocyte epitope in the mouse hepatitis virus nucleocapsid protein. J Virol. 1993 Dec;67(12):7041–7049. doi: 10.1128/jvi.67.12.7041-7049.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J., Sweetser M. T., Morrison L. A., Kittlesen D. J., Braciale V. L. Class I major histocompatibility complex-restricted cytolytic T lymphocytes recognize a limited number of sites on the influenza hemagglutinin. Proc Natl Acad Sci U S A. 1989 Jan;86(1):277–281. doi: 10.1073/pnas.86.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne H., Smith G., Beck S., Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature. 1990 Oct 25;347(6295):770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- Browning M. J., Huang A. S., Reiss C. S. Cytolytic T lymphocytes from the BALB/c-H-2dm2 mouse recognize the vesicular stomatitis virus glycoprotein and are restricted by class II MHC antigens. J Immunol. 1990 Aug 1;145(3):985–994. [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christinck E. R., Luscher M. A., Barber B. H., Williams D. B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991 Jul 4;352(6330):67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- Dveksler G. S., Pensiero M. N., Cardellichio C. B., Williams R. K., Jiang G. S., Holmes K. V., Dieffenbach C. W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991 Dec;65(12):6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A., Barnaba V., Natali P., Balsano C., Musca A., Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988 May-Jun;8(3):449–454. doi: 10.1002/hep.1840080302. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Ashwell J. D., Lechler R. I., Margulies D. H., Nickerson K. M., Suzuki G., Tou J. Y. "Exon-shuffling" maps control of antibody- and T-cell-recognition sites to the NH2-terminal domain of the class II major histocompatibility polypeptide A beta. Proc Natl Acad Sci U S A. 1985 May;82(9):2940–2944. doi: 10.1073/pnas.82.9.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Quill H. Unexpected expression of a unique mixed-isotype class II MHC molecule by transfected L-cells. Nature. 1986 Mar 6;320(6057):72–75. doi: 10.1038/320072a0. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Welsh R. M., Haspel M. V. Natural cytotoxicity against mouse hepatitis virus-infected target cells. I. Correlation of cytotoxicity with virus binding to leukocytes. J Immunol. 1986 Feb 15;136(4):1446–1453. [PubMed] [Google Scholar]
- Hou S., Doherty P. C., Zijlstra M., Jaenisch R., Katz J. M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992 Aug 15;149(4):1319–1325. [PubMed] [Google Scholar]
- Koolen M. J., Osterhaus A. D., Van Steenis G., Horzinek M. C., Van der Zeijst B. A. Temperature-sensitive mutants of mouse hepatitis virus strain A59: isolation, characterization and neuropathogenic properties. Virology. 1983 Mar;125(2):393–402. doi: 10.1016/0042-6822(83)90211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner H., Schliephake A., Winter J., Zimprich F., Lassmann H., Sedgwick J., Siddell S., Wege H. Nucleocapsid or spike protein-specific CD4+ T lymphocytes protect against coronavirus-induced encephalomyelitis in the absence of CD8+ T cells. J Immunol. 1991 Oct 1;147(7):2317–2323. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Minutello M. A., Pileri P., Unutmaz D., Censini S., Kuo G., Houghton M., Brunetto M. R., Bonino F., Abrignani S. Compartmentalization of T lymphocytes to the site of disease: intrahepatic CD4+ T cells specific for the protein NS4 of hepatitis C virus in patients with chronic hepatitis C. J Exp Med. 1993 Jul 1;178(1):17–25. doi: 10.1084/jem.178.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley J., Evans G., Dailey M. O., Perlman S. Immune response to a murine coronavirus: identification of a homing receptor-negative CD4+ T cell subset that responds to viral glycoproteins. Virology. 1992 Apr;187(2):443–452. doi: 10.1016/0042-6822(92)90446-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppler J. A., Nicholson J. K., Swan D. C., Ahmed-Ansari A., McDougal J. S. Down-modulation of MHC-I in a CD4+ T cell line, CEM-E5, after HIV-1 infection. J Immunol. 1989 Nov 1;143(9):2858–2866. [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Kyuwa S., Cohen M., Bergmann C., Polo J. M., Yeh J., Anthony R., Keck J. G. Mouse hepatitis virus nucleocapsid protein-specific cytotoxic T lymphocytes are Ld restricted and specific for the carboxy terminus. Virology. 1992 Jul;189(1):217–224. doi: 10.1016/0042-6822(92)90697-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Sussman M. A., Matsushima G. K., Shubin R. A., Erlich S. S. Delayed-type hypersensitivity response in the central nervous system during JHM virus infection requires viral specificity for protection. J Neuroimmunol. 1988 Sep;19(3):255–268. doi: 10.1016/0165-5728(88)90007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Vennema H., Heijnen L., Zijderveld A., Horzinek M. C., Spaan W. J. Intracellular transport of recombinant coronavirus spike proteins: implications for virus assembly. J Virol. 1990 Jan;64(1):339–346. doi: 10.1128/jvi.64.1.339-346.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., de Groot R. J., Harbour D. A., Horzinek M. C., Spaan W. J. Primary structure of the membrane and nucleocapsid protein genes of feline infectious peritonitis virus and immunogenicity of recombinant vaccinia viruses in kittens. Virology. 1991 Mar;181(1):327–335. doi: 10.1016/0042-6822(91)90499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Dörries R., Wege H. Hybridoma antibodies to the murine coronavirus JHM: characterization of epitopes on the peplomer protein (E2). J Gen Virol. 1984 Nov;65(Pt 11):1931–1942. doi: 10.1099/0022-1317-65-11-1931. [DOI] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Wysocka M., Korngold R., Yewdell J., Bennink J. Target and effector cell fusion accounts for B lymphocyte-mediated lysis of mouse hepatitis virus-infected cells. J Gen Virol. 1989 Jun;70(Pt 6):1465–1472. doi: 10.1099/0022-1317-70-6-1465. [DOI] [PubMed] [Google Scholar]
- van den Oord J. J., de Vos R., Desmet V. J. In situ distribution of major histocompatibility complex products and viral antigens in chronic hepatitis B virus infection: evidence that HBc-containing hepatocytes may express HLA-DR antigens. Hepatology. 1986 Sep-Oct;6(5):981–989. doi: 10.1002/hep.1840060529. [DOI] [PubMed] [Google Scholar]


