Abstract
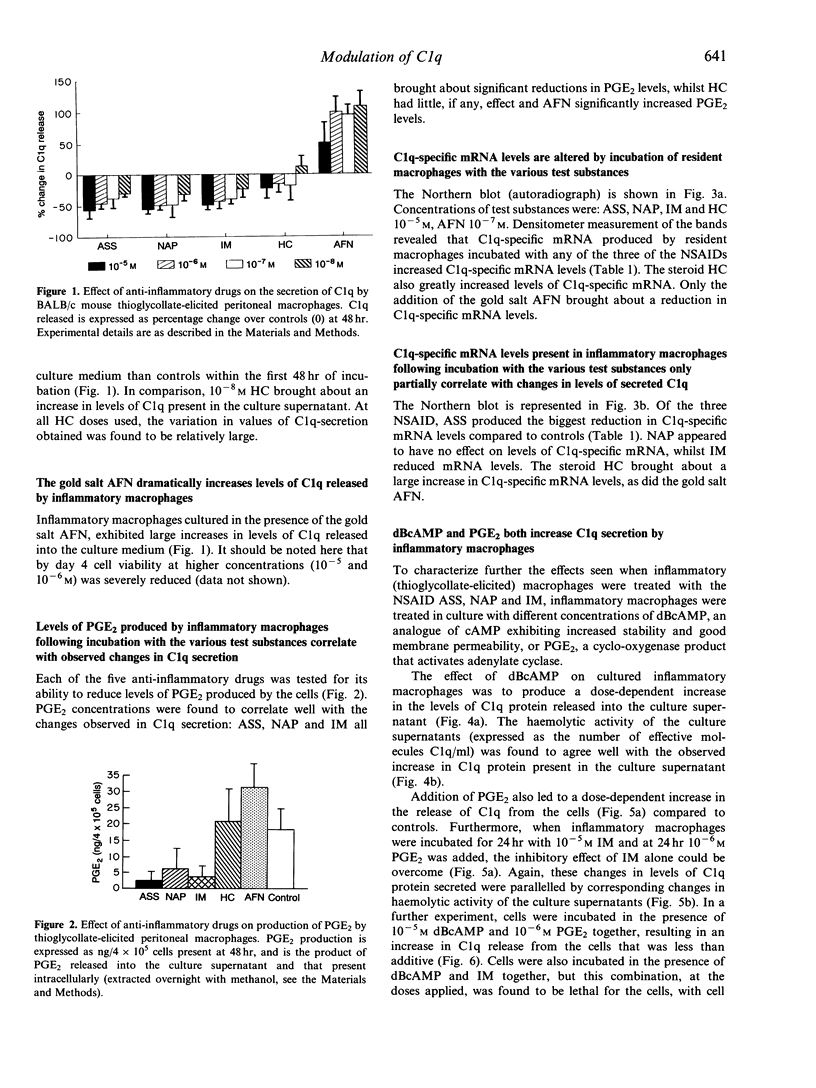
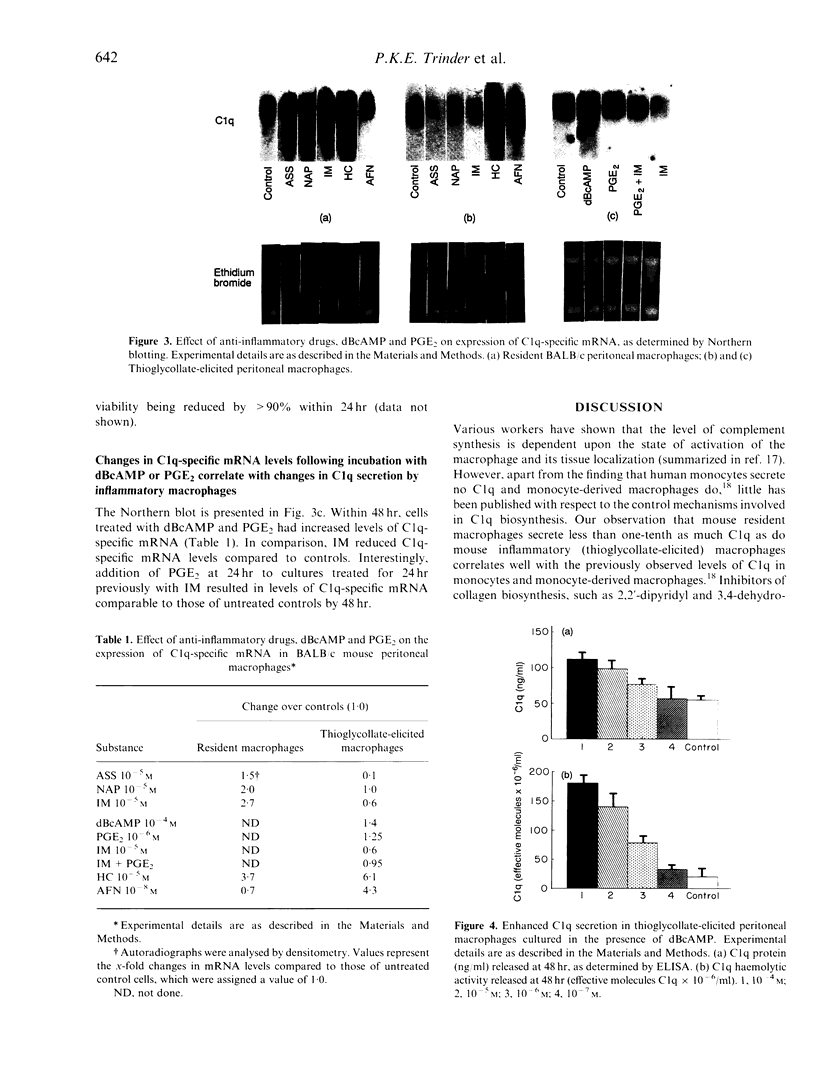
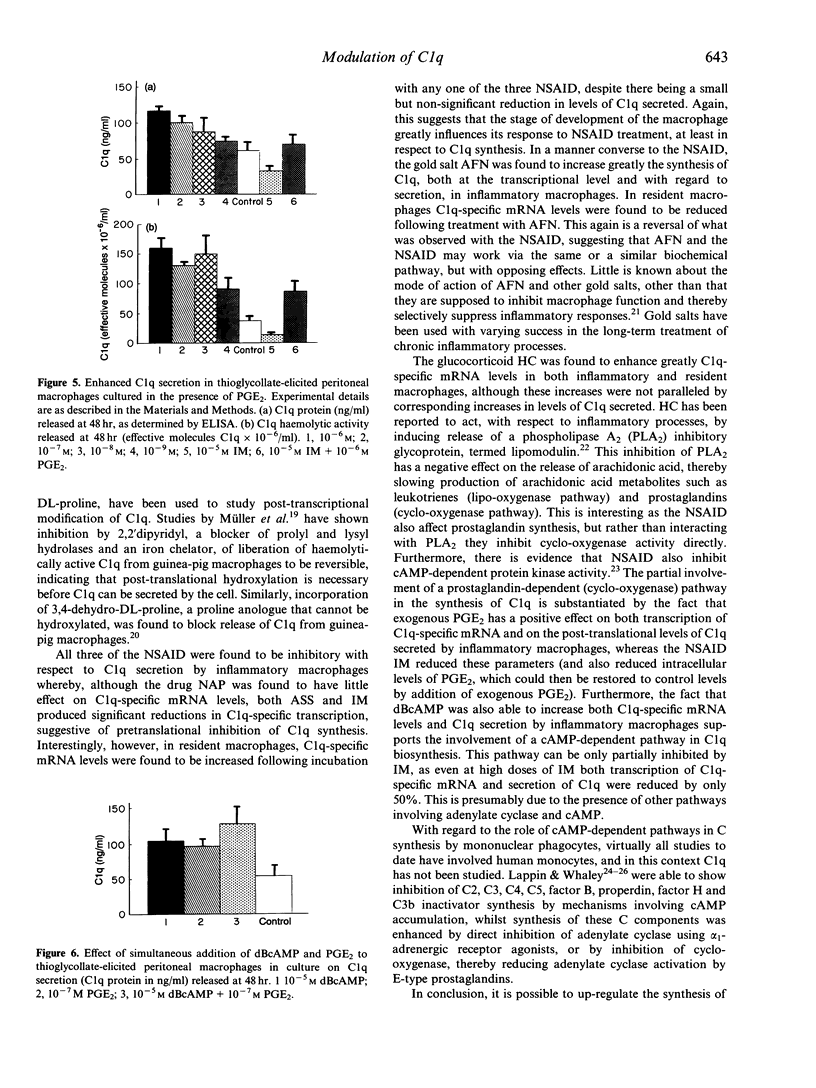
Isolated BALB/c mouse thioglycollate-elicited (inflammatory) peritoneal macrophages release at least 10 times more C1q than do isolated resident peritoneal macrophages. Addition of non-steroidal anti-inflammatory drugs (NSAID) to thioglycollate-elicited macrophages in culture inhibited the release of C1q and reduced levels of C1q-specific mRNA. Contrastingly, the NSAID were found to enhance C1q-specific mRNA levels in resident macrophages, although no increase in C1q levels secreted was observed. This suggests that the response of macrophages to NSAID, with respect to C1q synthesis, reflects the developmental stage of the macrophage. The gold salt auranofin (AFN) was found to enhance markedly C1q synthesis at both transcriptional and secretory levels in thioglycollate-elicited macrophages whilst, conversely, AFN reduced mRNA levels in resident macrophages. This indicates that AFN and the NSAID may work via the same or similar biochemical pathway, but with opposing effects. The glucocorticoid hydrocortisone (HC) greatly enhanced C1q-specific mRNA levels in both thioglycollate-elicited and resident macrophages, although no parallel increases in C1q secreted were observed. The data on inhibition of C1q biosynthesis by NSAID in thioglycollate-elicited macrophages are supported by the enhancement of C1q biosynthesis following addition of prostaglandin E2 (PGE2) or dibutyryl cyclic AMP (dBcAMP) to the cultures. From these experiments, it is concluded that C1q biosynthesis is controlled, at least in part, by a pathway involving cAMP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. B., Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1989 Jan;32(1):1–9. doi: 10.1002/anr.1780320102. [DOI] [PubMed] [Google Scholar]
- Bensa J. C., Reboul A., Colomb M. G. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem J. 1983 Nov 15;216(2):385–392. doi: 10.1042/bj2160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colten H. R., Strunk R. C., Perlmutter D. H., Cole F. S. Regulation of complement protein biosynthesis in mononuclear phagocytes. Ciba Found Symp. 1986;118:141–154. doi: 10.1002/9780470720998.ch10. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- Gewurz H., Ying S. C., Jiang H., Lint T. F. Nonimmune activation of the classical complement pathway. Behring Inst Mitt. 1993 Dec;(93):138–147. [PubMed] [Google Scholar]
- Golan M. D., Hitschold T., Loos M. The reconstitution of human C1, the first complement component: binding of C1r and C1s to C1q influences the C1q conformation. FEBS Lett. 1981 Jun 15;128(2):281–285. doi: 10.1016/0014-5793(81)80099-3. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Lappin D. F., Whaley K. Prostaglandins and prostaglandin synthetase inhibitors regulate the synthesis of complement components by human monocytes. Clin Exp Immunol. 1982 Sep;49(3):623–630. [PMC free article] [PubMed] [Google Scholar]
- Lappin D., Whaley K. Adrenergic receptors on monocytes modulate complement component synthesis. Clin Exp Immunol. 1982 Mar;47(3):606–612. [PMC free article] [PubMed] [Google Scholar]
- Lappin D., Whaley K. Cyclic AMP-mediated modulation of the production of the second component of human complement by monocytes. Int Arch Allergy Appl Immunol. 1981;65(1):85–90. doi: 10.1159/000232742. [DOI] [PubMed] [Google Scholar]
- Littman B. H., Hall R. E. Effects of gold sodium thiomalate on functional correlates of human monocyte maturation. Arthritis Rheum. 1985 Dec;28(12):1384–1392. doi: 10.1002/art.1780281211. [DOI] [PubMed] [Google Scholar]
- Loos M. The functions of endogenous C1q, a subcomponent of the first component of complement, as a receptor on the membrane of macrophages. Mol Immunol. 1982 Oct;19(10):1229–1238. doi: 10.1016/0161-5890(82)90288-7. [DOI] [PubMed] [Google Scholar]
- Mackenzie R., Coles G. A., Topley N., Powell W. S., Williams J. D. Down-regulation of cyclooxygenase product generation by human peritoneal macrophages. Immunology. 1992 Aug;76(4):648–654. [PMC free article] [PubMed] [Google Scholar]
- Minta J. O., Urowitz M. B., Smythe H. A., Isenman D. E. Effect on the human complement system of the major non-steroidal anti-inflammatory drugs: aspirin, indomethacin, phenylbutazone, oxyphenbutazone and sulindac. Clin Exp Immunol. 1983 Sep;53(3):555–561. [PMC free article] [PubMed] [Google Scholar]
- Müller W., Hanauske-Abel H., Loos M. Reversible inhibition of C1Q release from guinea pig macrophages by 2,2'-dipyridyl: Evidence for a posttranslational hydroxylation step in the biosynthesis of C1Q, a subcomponent of the first component of complement (C1). FEBS Lett. 1978 Jun 15;90(2):218–222. doi: 10.1016/0014-5793(78)80372-x. [DOI] [PubMed] [Google Scholar]
- Petry F., Reid K. B., Loos M. Gene expression of the A- and B-chain of mouse C1q in different tissues and the characterization of the recombinant A-chain. J Immunol. 1991 Dec 1;147(11):3988–3993. [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. Subunit composition and structure of subcomponent C1q of the first component of human complement. Biochem J. 1976 Apr 1;155(1):19–23. doi: 10.1042/bj1550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder P. K., Maeurer M. J., Kaul M., Petry E., Loos M. Functional domains of the human C1q A-chain. Behring Inst Mitt. 1993 Dec;(93):180–188. [PubMed] [Google Scholar]
- Watson W. C., Cremer M. A., Wooley P. H., Townes A. S. Assessment of the potential pathogenicity of type II collagen autoantibodies in patients with rheumatoid arthritis. Evidence of restricted IgG3 subclass expression and activation of complement C5 to C5a. Arthritis Rheum. 1986 Nov;29(11):1316–1321. doi: 10.1002/art.1780291103. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J. The first component of human complement (C1): activation and control. Springer Semin Immunopathol. 1983;6(2-3):213–230. doi: 10.1007/BF00205874. [DOI] [PubMed] [Google Scholar]



