Abstract
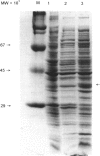
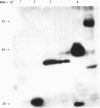
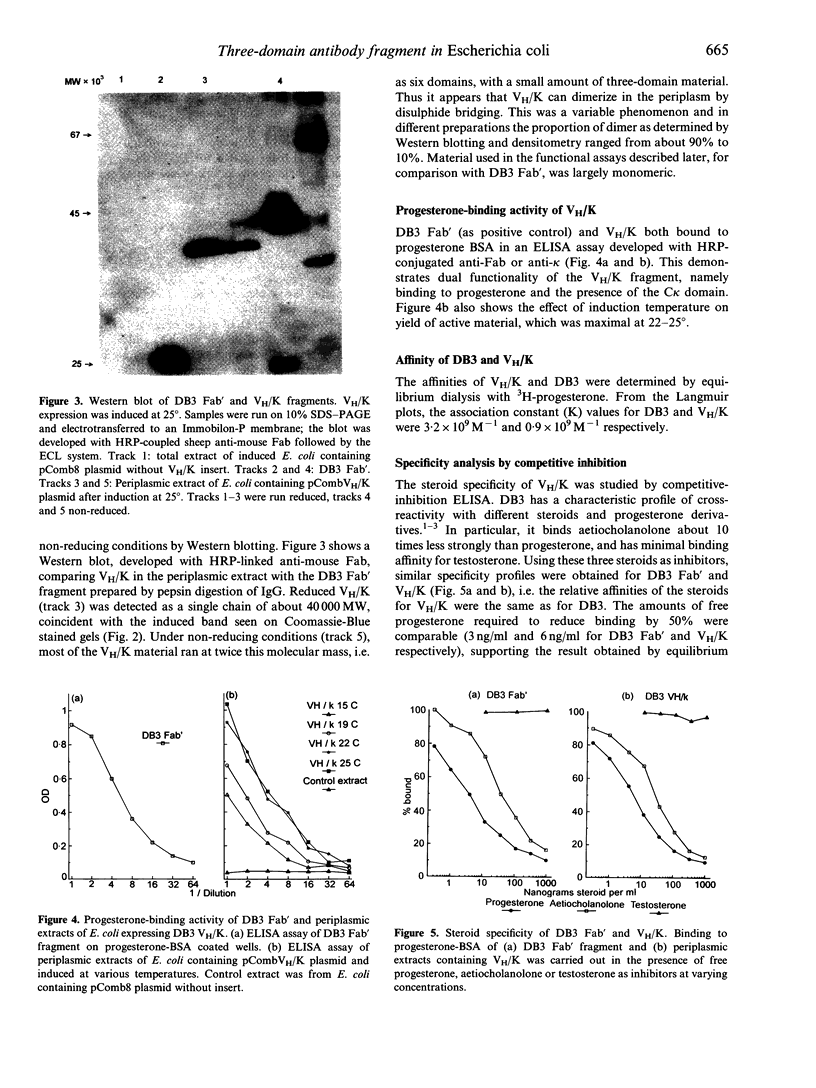
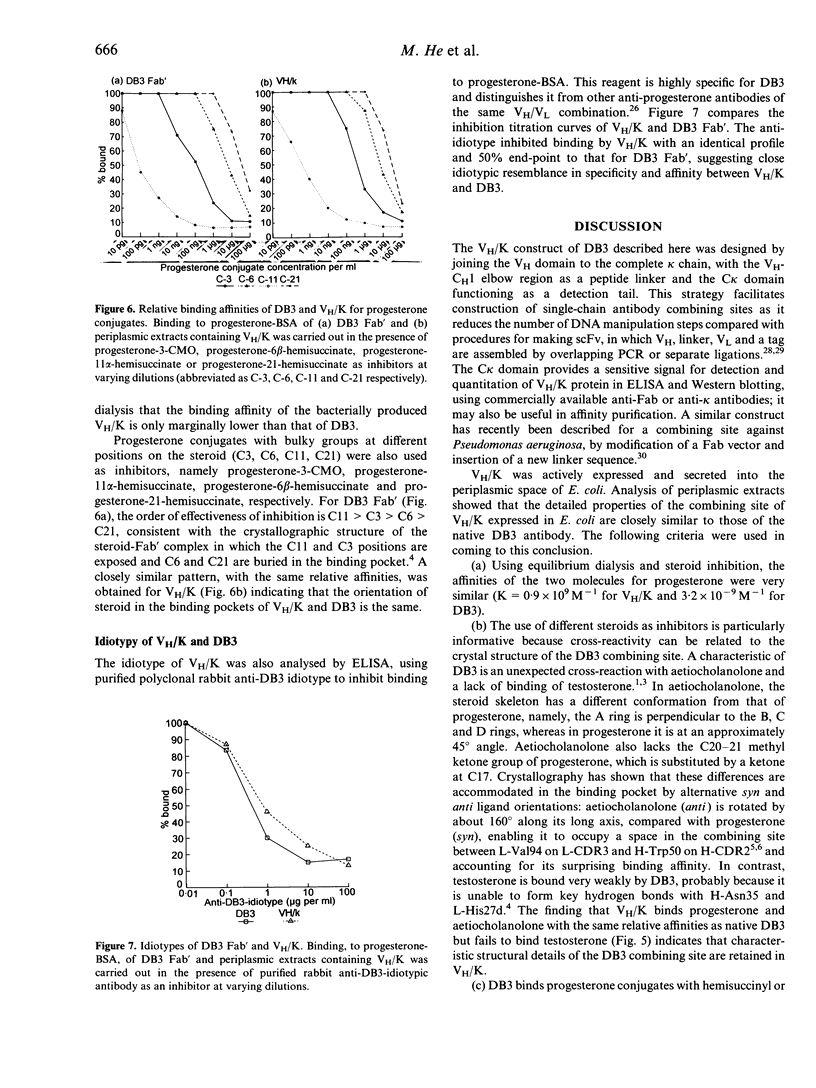
The heavy chain variable region (VH) and the kappa light chain of the anti-progesterone monoclonal antibody (mAb) DB3, have been expressed as a single-chain three-domain polypeptide, designated VH/K, and secreted into the periplasmic space of Escherichia coli (E. coli). The linker sequence was derived from the VH-CH1 elbow region. The C kappa domain provides a sensitive detection tail for Western blotting and enzyme-linked immunosorbent assay (ELISA). Periplasmic extracts of transformed E. coli contained material that bound progesterone and related steroids with similar specificity and affinity to DB3, and displayed the DB3 idiotype and kappa chain epitopes. Reference to the crystal structure of DB3 suggests that all the characteristics of the combining site interaction with steroids are retained in the bacterially expressed material. Western blotting demonstrated material with a molecular weight equivalent to three domains after reduction, but six domains in the unreduced state, suggesting that the VH/K polypeptide is assembled in the periplasm as a disulphide-bridged dimer. The VH/K construct provides a novel route to expression of antibody combining sites in E. coli for antibody engineering.
Full text
PDF
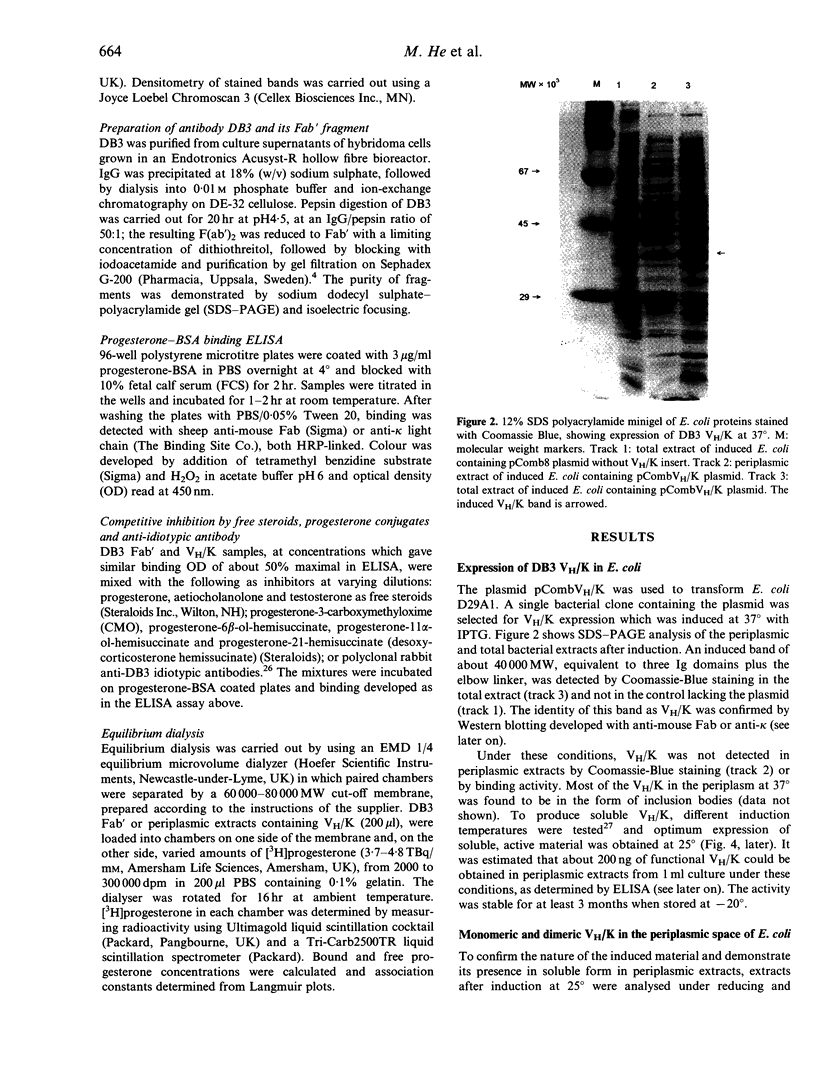


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand N. N., Mandal S., MacKenzie C. R., Sadowska J., Sigurskjold B., Young N. M., Bundle D. R., Narang S. A. Bacterial expression and secretion of various single-chain Fv genes encoding proteins specific for a Salmonella serotype B O-antigen. J Biol Chem. 1991 Nov 15;266(32):21874–21879. [PubMed] [Google Scholar]
- Arevalo J. H., Hassig C. A., Stura E. A., Sims M. J., Taussig M. J., Wilson I. A. Structural analysis of antibody specificity. Detailed comparison of five Fab'-steroid complexes. J Mol Biol. 1994 Sep 2;241(5):663–690. doi: 10.1006/jmbi.1994.1543. [DOI] [PubMed] [Google Scholar]
- Arevalo J. H., Stura E. A., Taussig M. J., Wilson I. A. Three-dimensional structure of an anti-steroid Fab' and progesterone-Fab' complex. J Mol Biol. 1993 May 5;231(1):103–118. doi: 10.1006/jmbi.1993.1260. [DOI] [PubMed] [Google Scholar]
- Arevalo J. H., Taussig M. J., Wilson I. A. Molecular basis of crossreactivity and the limits of antibody-antigen complementarity. Nature. 1993 Oct 28;365(6449):859–863. doi: 10.1038/365859a0. [DOI] [PubMed] [Google Scholar]
- Better M., Chang C. P., Robinson R. R., Horwitz A. H. Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988 May 20;240(4855):1041–1043. doi: 10.1126/science.3285471. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Carter P., Kelley R. F., Rodrigues M. L., Snedecor B., Covarrubias M., Velligan M. D., Wong W. L., Rowland A. M., Kotts C. E., Carver M. E. High level Escherichia coli expression and production of a bivalent humanized antibody fragment. Biotechnology (N Y) 1992 Feb;10(2):163–167. doi: 10.1038/nbt0292-163. [DOI] [PubMed] [Google Scholar]
- Cheadle C., Hook L. E., Givol D., Ricca G. A. Cloning and expression of the variable regions of mouse myeloma protein MOPC315 in E. coli: recovery of active FV fragments. Mol Immunol. 1992 Jan;29(1):21–30. doi: 10.1016/0161-5890(92)90152-n. [DOI] [PubMed] [Google Scholar]
- Colcher D., Bird R., Roselli M., Hardman K. D., Johnson S., Pope S., Dodd S. W., Pantoliano M. W., Milenic D. E., Schlom J. In vivo tumor targeting of a recombinant single-chain antigen-binding protein. J Natl Cancer Inst. 1990 Jul 18;82(14):1191–1197. doi: 10.1093/jnci/82.14.1191. [DOI] [PubMed] [Google Scholar]
- Deverson E., Berek C., Taussig M., Feinstein A. Monoclonal BALB/c anti-progesterone antibodies use family IX variable region heavy chain genes. Eur J Immunol. 1987 Jan;17(1):9–13. doi: 10.1002/eji.1830170103. [DOI] [PubMed] [Google Scholar]
- Ellis S. T., Heap R. B., Butchart A. R., Rider V., Richardson N. E., Wang M. W., Taussig M. J. Efficacy and specificity of monoclonal antibodies to progesterone in preventing the establishment of pregnancy in the mouse. J Endocrinol. 1988 Jul;118(1):69–80. doi: 10.1677/joe.0.1180069. [DOI] [PubMed] [Google Scholar]
- Gandecha A. R., Owen M. R., Cockburn B., Whitelam G. C. Production and secretion of a bifunctional staphylococcal protein A::antiphytochrome single-chain Fv fusion protein in Escherichia coli. Gene. 1992 Dec 15;122(2):361–365. doi: 10.1016/0378-1119(92)90227-g. [DOI] [PubMed] [Google Scholar]
- Glockshuber R., Malia M., Pfitzinger I., Plückthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990 Feb 13;29(6):1362–1367. doi: 10.1021/bi00458a002. [DOI] [PubMed] [Google Scholar]
- Gram H., Marconi L. A., Barbas C. F., 3rd, Collet T. A., Lerner R. A., Kang A. S. In vitro selection and affinity maturation of antibodies from a naive combinatorial immunoglobulin library. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3576–3580. doi: 10.1073/pnas.89.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Wilde A., Kaderbhai M. A. A simple single-step procedure for small-scale preparation of Escherichia coli plasmids. Nucleic Acids Res. 1990 Mar 25;18(6):1660–1660. doi: 10.1093/nar/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger P., Prospero T., Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappik A., Krebber C., Plückthun A. The effect of folding catalysts on the in vivo folding process of different antibody fragments expressed in Escherichia coli. Biotechnology (N Y) 1993 Jan;11(1):77–83. doi: 10.1038/nbt0193-77. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McGregor D. P., Molloy P. E., Cunningham C., Harris W. J. Spontaneous assembly of bivalent single chain antibody fragments in Escherichia coli. Mol Immunol. 1994 Feb;31(3):219–226. doi: 10.1016/0161-5890(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Pack P., Kujau M., Schroeckh V., Knüpfer U., Wenderoth R., Riesenberg D., Plückthun A. Improved bivalent miniantibodies, with identical avidity as whole antibodies, produced by high cell density fermentation of Escherichia coli. Biotechnology (N Y) 1993 Nov;11(11):1271–1277. doi: 10.1038/nbt1193-1271. [DOI] [PubMed] [Google Scholar]
- Pack P., Plückthun A. Miniantibodies: use of amphipathic helices to produce functional, flexibly linked dimeric FV fragments with high avidity in Escherichia coli. Biochemistry. 1992 Feb 18;31(6):1579–1584. doi: 10.1021/bi00121a001. [DOI] [PubMed] [Google Scholar]
- Plückthun A. Mono- and bivalent antibody fragments produced in Escherichia coli: engineering, folding and antigen binding. Immunol Rev. 1992 Dec;130:151–188. doi: 10.1111/j.1600-065x.1992.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Schein C. H. Optimizing protein folding to the native state in bacteria. Curr Opin Biotechnol. 1991 Oct;2(5):746–750. doi: 10.1016/0958-1669(91)90046-8. [DOI] [PubMed] [Google Scholar]
- Sims M. J., Krawinkel U., Taussig M. J. Characterization of germ-line genes of the VGAM3.8 VH gene family from BALB/c mice. J Immunol. 1992 Sep 1;149(5):1642–1648. [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Secretion and in vivo folding of the Fab fragment of the antibody McPC603 in Escherichia coli: influence of disulphides and cis-prolines. Protein Eng. 1991 Dec;4(8):971–979. doi: 10.1093/protein/4.8.971. [DOI] [PubMed] [Google Scholar]
- Stura E. A., Arevalo J. H., Feinstein A., Heap R. B., Taussig M. J., Wilson I. A. Analysis of an anti-progesterone antibody: variable crystal morphology of the Fab' and steroid-Fab' complexes. Immunology. 1987 Dec;62(4):511–521. [PMC free article] [PubMed] [Google Scholar]
- Tai M. S., Mudgett-Hunter M., Levinson D., Wu G. M., Haber E., Oppermann H., Huston J. S. A bifunctional fusion protein containing Fc-binding fragment B of staphylococcal protein A amino terminal to antidigoxin single-chain Fv. Biochemistry. 1990 Sep 4;29(35):8024–8030. doi: 10.1021/bi00487a005. [DOI] [PubMed] [Google Scholar]
- Taussig M. J., Brown N., Ellis S., Holliman A., Peat D., Richardson N., Heap R. B., Feinstein A. Anti-idiotypic sera against monoclonal anti-progesterone antibodies: production in rabbits and rats and characterization of specificity. Immunology. 1986 Jul;58(3):445–452. [PMC free article] [PubMed] [Google Scholar]
- Tavladoraki P., Benvenuto E., Trinca S., De Martinis D., Cattaneo A., Galeffi P. Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature. 1993 Dec 2;366(6454):469–472. doi: 10.1038/366469a0. [DOI] [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- Wels W., Harwerth I. M., Zwickl M., Hardman N., Groner B., Hynes N. E. Construction, bacterial expression and characterization of a bifunctional single-chain antibody-phosphatase fusion protein targeted to the human erbB-2 receptor. Biotechnology (N Y) 1992 Oct;10(10):1128–1132. doi: 10.1038/nbt1092-1128. [DOI] [PubMed] [Google Scholar]
- Wright L. J., Feinstein A., Heap R. B., Saunders J. C., Bennett R. C., Wang M. Y. Progesterone monoclonal antibody blocks pregnancy in mice. Nature. 1982 Feb 4;295(5848):415–417. doi: 10.1038/295415a0. [DOI] [PubMed] [Google Scholar]