Abstract
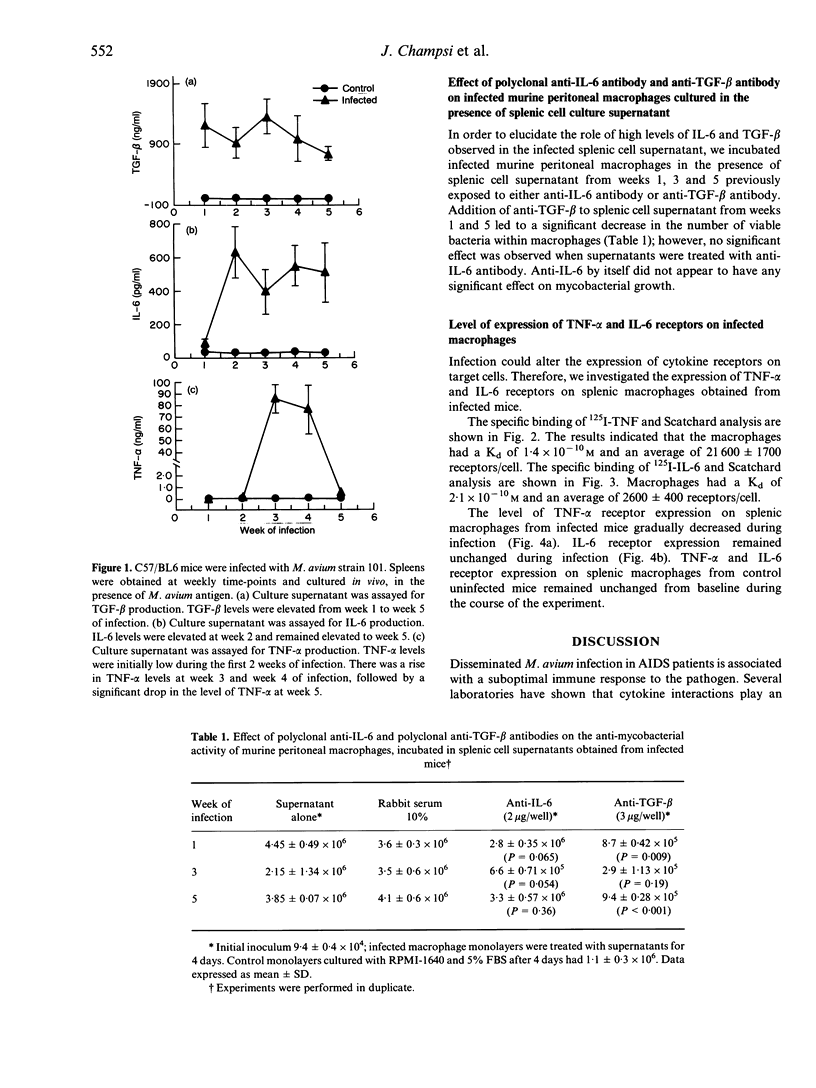
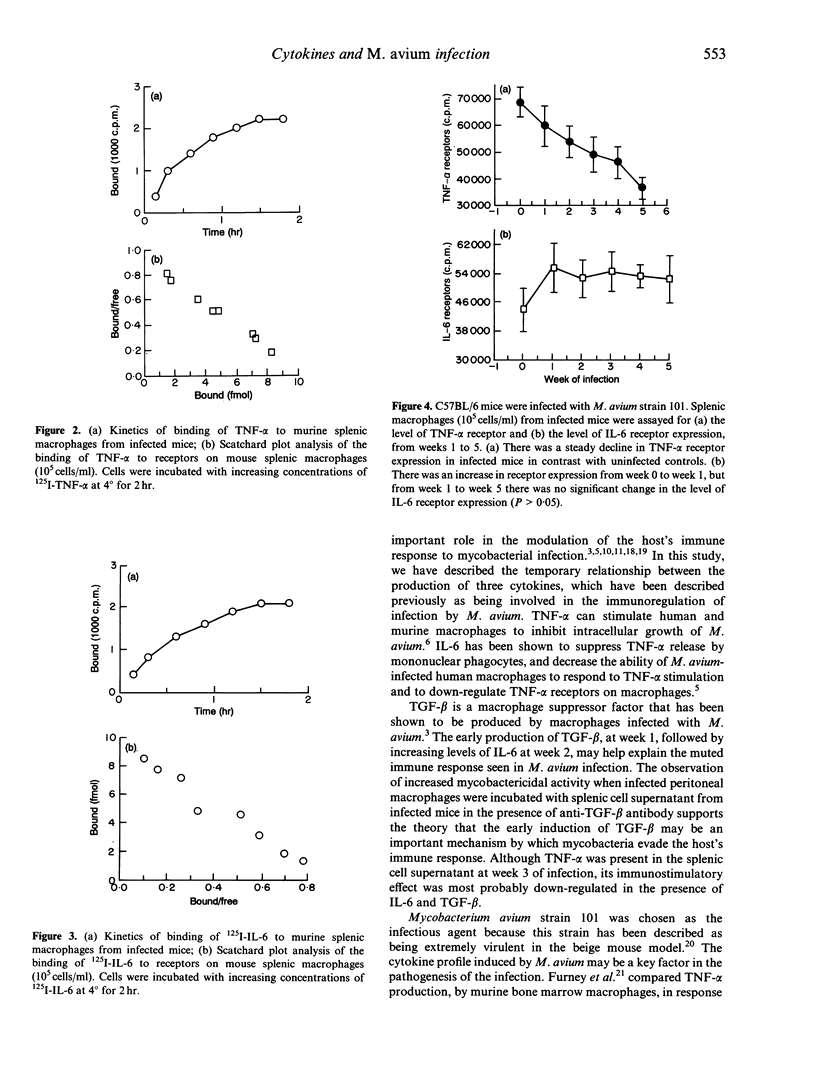
The Mycobacterium avium complex comprises intracellular bacteria associated with disseminated infection in patients with acquired immune deficiency syndrome (AIDS). Immune defects that lead to infection are unknown but cytokines appear to play an important role in the immunomodulation of host defence mechanisms. We evaluated the cytokine profiles seen temporally after murine M. avium infection. Spleen cells were obtained from M. avium-infected C57BL/6 mice and uninfected mice at weeks 1, 2, 3, 4 and 5. Cells were cultured in vitro and subsequently pulsed with killed M. avium. Supernatants were collected from the cultured splenic cells and the concentrations of interleukin-6 (IL-6), transforming growth factor-beta 1 (TGF-beta 1) and tumour necrosis factor-alpha (TNF-alpha) were measured. TGF-beta 1 was detected at week 1, followed by IL-6 production at week 2. Elevated TNF-alpha levels were observed at week 3. The addition of polyclonal anti-TGF-beta 1 antibody to M. avium-infected peritoneal macrophages in the presence of splenic cell supernatants from weeks 1, 3 and 5 led to decreased bacterial counts compared to controls. Anti-IL-6 antibody did not have any effect on macrophage anti-mycobacterial activity. Concurrently, we observed decreased expression of TNF-alpha receptors on infected macrophages. We propose that the early elevated levels of TGF-beta 1, a known suppressor of macrophage function, in conjunction with down-regulation of TNF-alpha receptors may help explain the suboptimal macrophage response to TNF-alpha, leading to impaired anti-mycobacterial activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barral-Netto M., Barral A., Brownell C. E., Skeiky Y. A., Ellingsworth L. R., Twardzik D. R., Reed S. G. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992 Jul 24;257(5069):545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993 Jul;61(7):3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Covaro G., Remington J. Infection of murine macrophages with Toxoplasma gondii is associated with release of transforming growth factor beta and downregulation of expression of tumor necrosis factor receptors. Infect Immun. 1993 Oct;61(10):4126–4130. doi: 10.1128/iai.61.10.4126-4130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J Immunol. 1993 Mar 1;150(5):1838–1845. [PubMed] [Google Scholar]
- Bermudez L. E., Stevens P., Kolonoski P., Wu M., Young L. S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol. 1989 Nov 1;143(9):2996–3000. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Petrofsky M., Young L. S. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992 Oct;60(10):4245–4252. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Gupta S. 1,25 Dihydroxyvitamin D3-dependent inhibition of growth or killing of Mycobacterium avium complex in human macrophages is mediated by TNF and GM-CSF. Cell Immunol. 1990 May;127(2):432–441. doi: 10.1016/0008-8749(90)90144-g. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Bertram M. A., Inderlied C. B., Yadegar S., Kolanoski P., Yamada J. K., Young L. S. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J Infect Dis. 1986 Jul;154(1):194–195. doi: 10.1093/infdis/154.1.194. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Pearson C. A., Freitag C. S., Djeu J. Y. Mycobacterium avium-intracellulare induces interleukin-6 from human monocytes and large granular lymphocytes. Blood. 1991 May 15;77(10):2218–2224. [PubMed] [Google Scholar]
- De Titto E. H., Catterall J. R., Remington J. S. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986 Aug 15;137(4):1342–1345. [PubMed] [Google Scholar]
- De Titto E. H., Catterall J. R., Remington J. S. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986 Aug 15;137(4):1342–1345. [PubMed] [Google Scholar]
- Denis M., Ghadirian E. Transforming growth factor beta (TGF-b1) plays a detrimental role in the progression of experimental Mycobacterium avium infection; in vivo and in vitro evidence. Microb Pathog. 1991 Nov;11(5):367–372. doi: 10.1016/0882-4010(91)90022-3. [DOI] [PubMed] [Google Scholar]
- Denis M., Gregg E. O. Recombinant tumour necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990 Sep;71(1):139–141. [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interleukin-6 is used as a growth factor by virulent Mycobacterium avium: presence of specific receptors. Cell Immunol. 1992 Apr 15;141(1):182–188. doi: 10.1016/0008-8749(92)90137-e. [DOI] [PubMed] [Google Scholar]
- Furney S. K., Skinner P. S., Roberts A. D., Appelberg R., Orme I. M. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect Immun. 1992 Oct;60(10):4410–4413. doi: 10.1128/iai.60.10.4410-4413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähdevirta J., Maury C. P., Teppo A. M., Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988 Sep;85(3):289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Ellner J. J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991 May 1;146(9):3165–3170. [PubMed] [Google Scholar]
- Silva J. S., Twardzik D. R., Reed S. G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J Exp Med. 1991 Sep 1;174(3):539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]



