Abstract
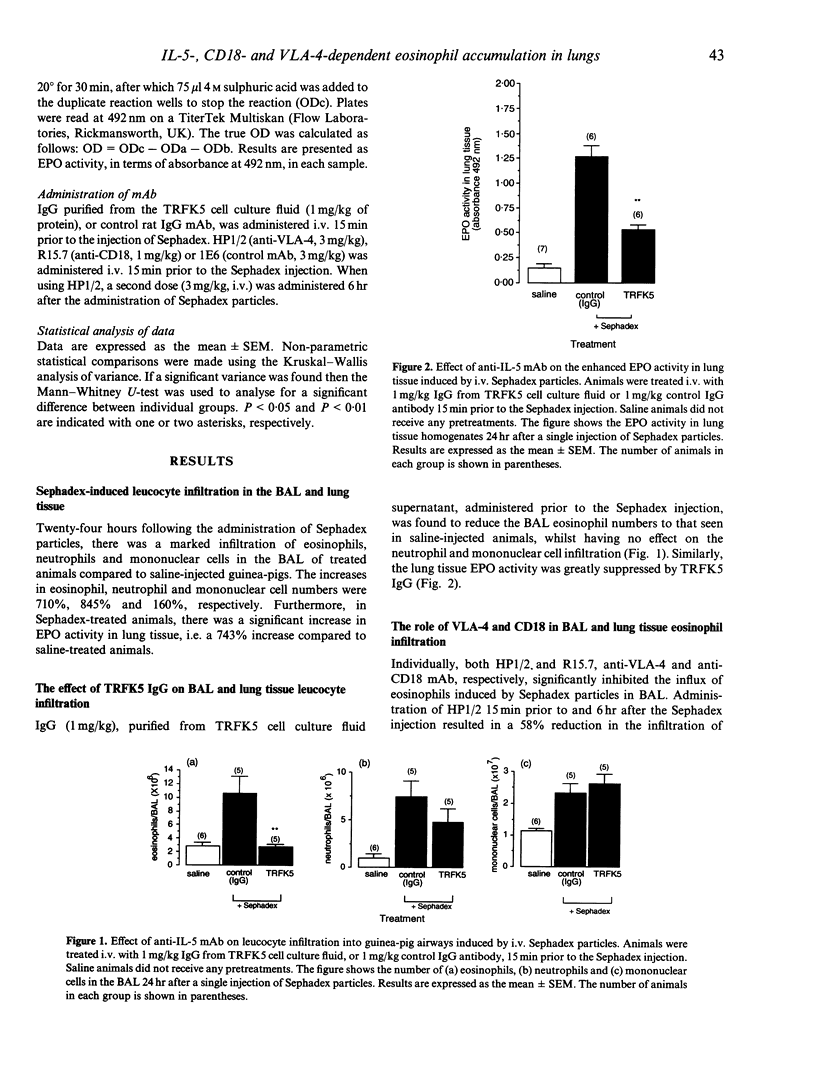
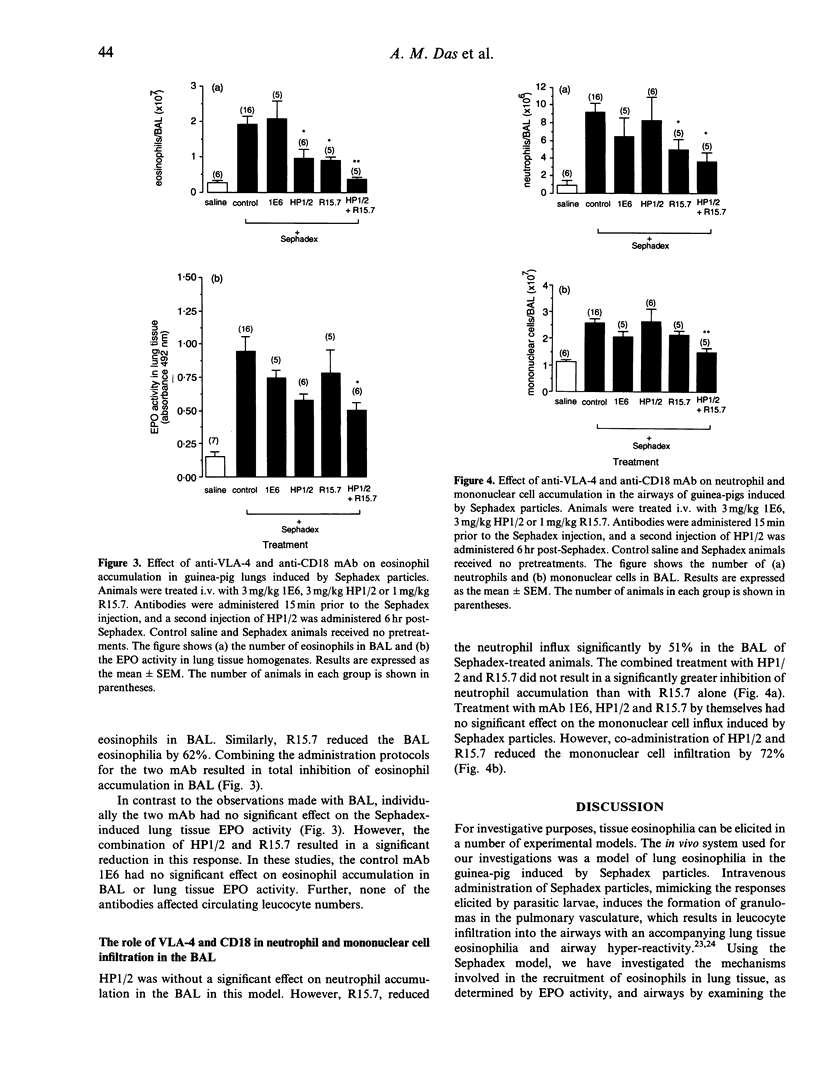
Blood and tissue-eosinophilia is a characteristic feature of a number of disease states. In experimental animals, the intravenous injection of parasitic larvae induces a profound eosinophilia that can be mimicked by the intravenous injection of Sephadex particles. In the present study, this procedure was used to investigate the mechanisms involved in the development of lung eosinophilia in a guinea-pig model. Intravenous administration of Sephadex particles to guinea-pigs resulted in a significant increase in the influx of eosinophils in the airways and in lung tissue eosinophil peroxidase (EPO) activity (at t = 24 hr). An anti-interleukin-5 (IL-5) monoclonal antibody (mAb) totally inhibited the eosinophilia in the airways and significantly reduced the lung tissue EPO activity. The concomitant accumulation of neutrophils and mononuclear cells, however, was not affected by this treatment. Monoclonal antibodies to VLA-4 and CD18 caused 58% and 62% suppression of eosinophilia in the bronchoalveolar lavage (BAL), respectively, whilst having no effect on lung tissue EPO activity. Co-administration of the two mAb resulted in total inhibition of eosinophil accumulation into BAL and significant suppression of lung tissue EPO activity (55% inhibition). This procedure also resulted in 72% inhibition of mononuclear cell influx and 68% inhibition of neutrophil influx in the BAL, the latter effect being entirely due to the actions of the anti-CD18 mAb. The results of this study indicate for the first time a requirement for IL-5 in the development of lung eosinophilia in this model. Further, it is clear that both the molecules VLA-4 and CD18 contribute to the development of this response and that maximal inhibition of lung eosinophilia is achieved only when the two adhesion pathways are simultaneously blocked.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991 Jun 1;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Cheng J. B., Pillar J. S., Shirley J. T., Showell H. J., Watson J. W., Cohan V. L. Antigen-mediated pulmonary eosinophilia in immunoglobulin G1-sensitized guinea pigs: eosinophil peroxidase as a simple specific marker for detecting eosinophils in bronchoalveolar lavage fluid. J Pharmacol Exp Ther. 1993 Feb;264(2):922–929. [PubMed] [Google Scholar]
- Cook R. M. Eosinophil accumulation in rats injected with Sephadex particles. Clin Exp Allergy. 1990 Sep;20(5):511–517. doi: 10.1111/j.1365-2222.1990.tb03143.x. [DOI] [PubMed] [Google Scholar]
- Cook R. M., Musgrove N. R., Smith H. Eosinophils and the granulomatous reaction in rats injected with Sephadex particles. Pulm Pharmacol. 1989;2(4):185–190. doi: 10.1016/0952-0600(89)90019-7. [DOI] [PubMed] [Google Scholar]
- Denburg J. A., Gauldie J., Dolovich J., Ohtoshi T., Cox G., Jordana M. Structural cell-derived cytokines in allergic inflammation. Int Arch Allergy Appl Immunol. 1991;94(1-4):127–132. doi: 10.1159/000235343. [DOI] [PubMed] [Google Scholar]
- Dobrina A., Menegazzi R., Carlos T. M., Nardon E., Cramer R., Zacchi T., Harlan J. M., Patriarca P. Mechanisms of eosinophil adherence to cultured vascular endothelial cells. Eosinophils bind to the cytokine-induced ligand vascular cell adhesion molecule-1 via the very late activation antigen-4 integrin receptor. J Clin Invest. 1991 Jul;88(1):20–26. doi: 10.1172/JCI115278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerschuk C. M., Winn R. K., Coxson H. O., Harlan J. M. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990 Mar 15;144(6):2327–2333. [PubMed] [Google Scholar]
- Ebisawa M., Bochner B. S., Georas S. N., Schleimer R. P. Eosinophil transendothelial migration induced by cytokines. I. Role of endothelial and eosinophil adhesion molecules in IL-1 beta-induced transendothelial migration. J Immunol. 1992 Dec 15;149(12):4021–4028. [PubMed] [Google Scholar]
- Griffiths-Johnson D. A., Collins P. D., Rossi A. G., Jose P. J., Williams T. J. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993 Dec 30;197(3):1167–1172. doi: 10.1006/bbrc.1993.2599. [DOI] [PubMed] [Google Scholar]
- Gulbenkian A. R., Egan R. W., Fernandez X., Jones H., Kreutner W., Kung T., Payvandi F., Sullivan L., Zurcher J. A., Watnick A. S. Interleukin-5 modulates eosinophil accumulation in allergic guinea pig lung. Am Rev Respir Dis. 1992 Jul;146(1):263–266. doi: 10.1164/ajrccm/146.1.263. [DOI] [PubMed] [Google Scholar]
- Hakkert B. C., Kuijpers T. W., Leeuwenberg J. F., van Mourik J. A., Roos D. Neutrophil and monocyte adherence to and migration across monolayers of cytokine-activated endothelial cells: the contribution of CD18, ELAM-1, and VLA-4. Blood. 1991 Nov 15;78(10):2721–2726. [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyoshi Y., Dörschner A., Mallet A. I., Christophers E., Schröder J. M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992 Aug 1;176(2):587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Corrigan C. J. Asthma. Eosinophils and neutrophils. Br Med Bull. 1992 Jan;48(1):51–64. doi: 10.1093/oxfordjournals.bmb.a072541. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy G., Liu M. C., Su S. N., Kumai M., Xiao H. Q., Marsh D. G., Huang S. K. Analysis of cytokine transcripts in the bronchoalveolar lavage cells of patients with asthma. Am J Respir Cell Mol Biol. 1993 Sep;9(3):279–286. doi: 10.1165/ajrcmb/9.3.279. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., Mul E. P., Blom M., Kovach N. L., Gaeta F. C., Tollefson V., Elices M. J., Harlan J. M. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993 Jul 1;178(1):279–284. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock S. M., Smith H., Spicer B. A. Airway hyper-reactivity and blood, lung and airway eosinophilia in rats treated with Sephadex particles. Int Arch Allergy Appl Immunol. 1986;81(4):363–367. doi: 10.1159/000234164. [DOI] [PubMed] [Google Scholar]
- Mauser P. J., Pitman A., Witt A., Fernandez X., Zurcher J., Kung T., Jones H., Watnick A. S., Egan R. W., Kreutner W. Inhibitory effect of the TRFK-5 anti-IL-5 antibody in a guinea pig model of asthma. Am Rev Respir Dis. 1993 Dec;148(6 Pt 1):1623–1627. doi: 10.1164/ajrccm/148.6_Pt_1.1623. [DOI] [PubMed] [Google Scholar]
- Nogueira de Francischi J., Conroy D. M., Maghni K., Sirois P. Inhibition by rapamycin of leukocyte migration and bronchial hyperreactivity induced by injection of Sephadex beads to guinea-pigs. Br J Pharmacol. 1993 Dec;110(4):1381–1386. doi: 10.1111/j.1476-5381.1993.tb13973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan T. C., Gundel R. H., Desai S. N., Stearns C., Barton R. W., Rothlein R., Letts L. G., Piper P. J. The effects of an anti-CD18 antibody (R15.7) in antigen-induced airway hyperresponsiveness (AH) and cell influx in guinea pigs. Agents Actions. 1991 Sep;34(1-2):211–213. doi: 10.1007/BF01993282. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J. Interleukin-5, eosinophils, and disease. Blood. 1992 Jun 15;79(12):3101–3109. [PubMed] [Google Scholar]
- Sehmi R., Wardlaw A. J., Cromwell O., Kurihara K., Waltmann P., Kay A. B. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992 Jun 1;79(11):2952–2959. [PubMed] [Google Scholar]
- Spicer B. A., Baker R., Laycock S. M., Smith H. Correlation between blood eosinophilia and airways hyper-responsiveness in rats. Agents Actions. 1989 Jan;26(1-2):63–65. [PubMed] [Google Scholar]
- Strath M., Warren D. J., Sanderson C. J. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985 Nov 7;83(2):209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout A. J., Ladenius A. R., Savelkoul H. F., Van Ark I., Delsman K. C., Nijkamp F. P. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993 Mar;147(3):548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- Walls R. S., Beeson P. B. Mechanism of eosinophilia. IX. Induction of eosinophilia in rats by certain forms of dextran. Proc Soc Exp Biol Med. 1972 Jun;140(2):689–693. doi: 10.3181/00379727-140-36532. [DOI] [PubMed] [Google Scholar]
- Walsh G. M., Mermod J. J., Hartnell A., Kay A. B., Wardlaw A. J. Human eosinophil, but not neutrophil, adherence to IL-1-stimulated human umbilical vascular endothelial cells is alpha 4 beta 1 (very late antigen-4) dependent. J Immunol. 1991 May 15;146(10):3419–3423. [PubMed] [Google Scholar]
- Wang J. M., Rambaldi A., Biondi A., Chen Z. G., Sanderson C. J., Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989 Apr;19(4):701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Moqbel R., Cromwell O., Kay A. B. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986 Dec;78(6):1701–1706. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warringa R. A., Schweizer R. C., Maikoe T., Kuijper P. H., Bruijnzeel P. L., Koendermann L. Modulation of eosinophil chemotaxis by interleukin-5. Am J Respir Cell Mol Biol. 1992 Dec;7(6):631–636. doi: 10.1165/ajrcmb/7.6.631. [DOI] [PubMed] [Google Scholar]
- Weg V. B., Williams T. J., Lobb R. R., Nourshargh S. A monoclonal antibody recognizing very late activation antigen-4 inhibits eosinophil accumulation in vivo. J Exp Med. 1993 Feb 1;177(2):561–566. doi: 10.1084/jem.177.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Rand T. H., Goelz S. E., Chi-Rosso G., Lobb R. R. Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7430–7433. doi: 10.1073/pnas.88.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]


