Abstract
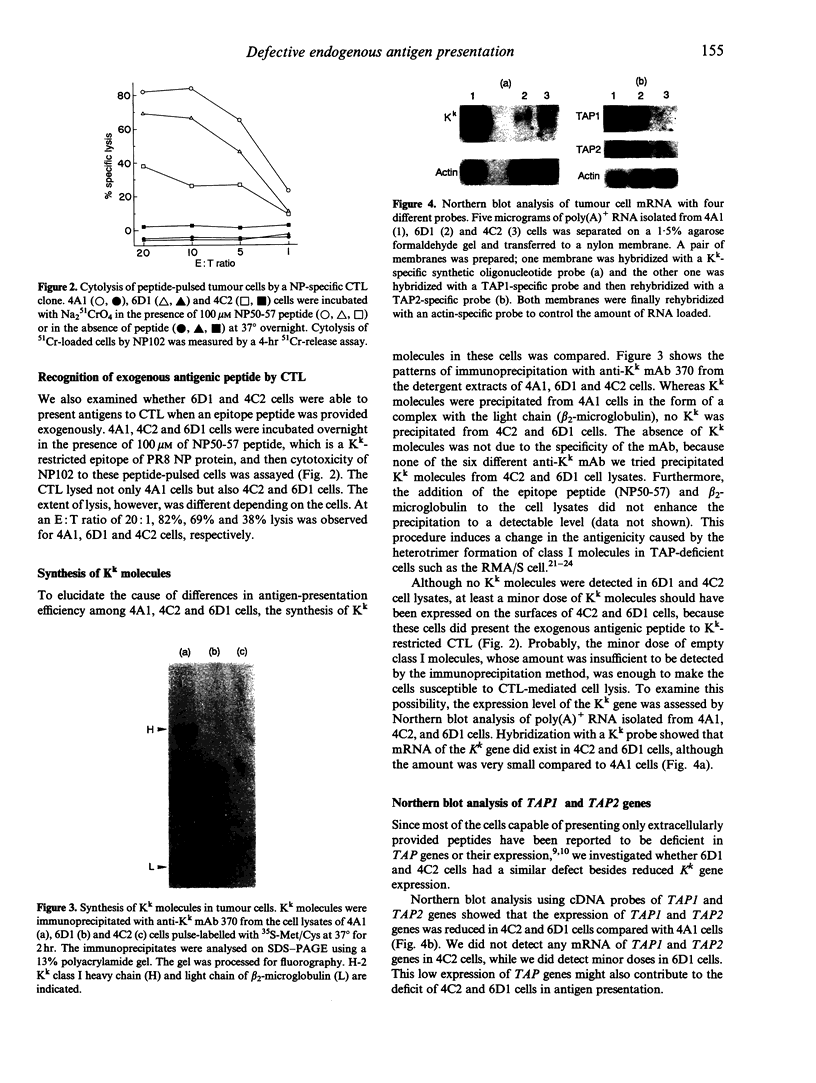
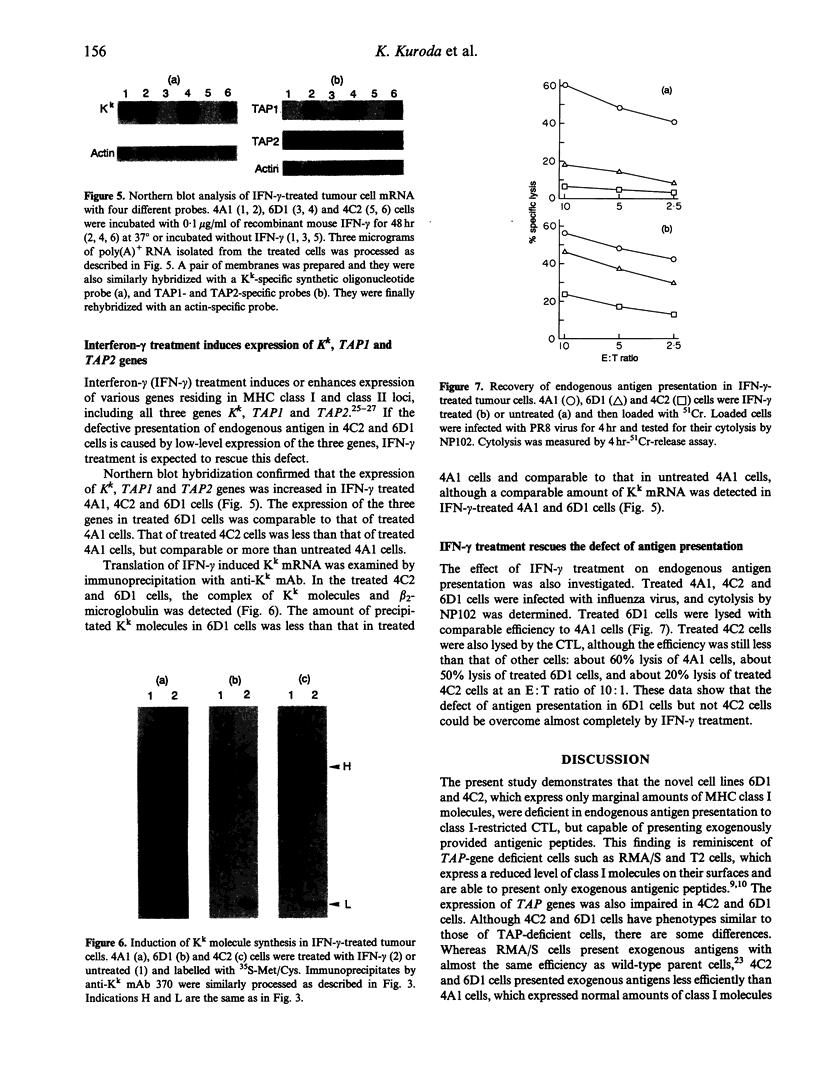
Three cell lines (4A1, 4C2 and 6D1 cells) derived from fibrosarcoma induced by the inoculation of 3-methylcholanthrene into C3H/HeN (H-2k) mice were examined for their ability to present antigens to CD8+ cytotoxic T lymphocytes (CTL). 6D1 and 4C2 cells were deficient in presenting endogenously synthesized influenza virus antigens to CTL, but they were able to present antigens when they were sensitized with a synthetic epitope peptide. The expression of the H-2 Kk gene in 4C2 and 6D1 cells was much reduced and was detectable only with Northern blot hybridization. The expression of two transporter genes (TAP1 and TAP2), examined by Northern hybridization, was also reduced in both cells, and negligible particularly in 4C2 cells. Interferon-gamma (IFN-gamma) treatment of these cells induced expression of Kk, TAP1 and TAP2 genes and rescued the defect of class I-restricted antigen presentation in 4C2 and 6D1 cells. Even after this treatment, however, antigen-presentation capability of 4C2 cells was still much lower than that of normal 4A1 cells. This finding suggests that 4C2 cells might have an additional defective gene(s), whose products are involved in the processing of class I-restricted antigen, besides the Kk and TAP genes, and this may explain the difficulty of 4C2 cells to induce tumour-specific immunity, as described previously. To our knowledge, the 4C2 cell is the first tumour cell postulated to have more than three defective genes involved in class I-restricted antigen presentation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold D., Driscoll J., Androlewicz M., Hughes E., Cresswell P., Spies T. Proteasome subunits encoded in the MHC are not generally required for the processing of peptides bound by MHC class I molecules. Nature. 1992 Nov 12;360(6400):171–174. doi: 10.1038/360171a0. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Alexander J., Anderson K., Lamb C., Cresswell P., McMichael A., Gotch F., Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990 May 31;345(6274):449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- Cossins J., Gould K., Brownlee G. G. Peptides shorter than a minimal CTL epitope may have a higher binding affinity than the epitope for the class I Kk molecule. Virology. 1993 Aug;195(2):851–854. doi: 10.1006/viro.1993.1443. [DOI] [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll J., Brown M. G., Finley D., Monaco J. J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993 Sep 16;365(6443):262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- Elliott B. E., Carlow D. A., Rodricks A. M., Wade A. Perspectives on the role of MHC antigens in normal and malignant cell development. Adv Cancer Res. 1989;53:181–245. doi: 10.1016/s0065-230x(08)60282-1. [DOI] [PubMed] [Google Scholar]
- Elliott T., Cerundolo V., Elvin J., Townsend A. Peptide-induced conformational change of the class I heavy chain. Nature. 1991 May 30;351(6325):402–406. doi: 10.1038/351402a0. [DOI] [PubMed] [Google Scholar]
- Gaczynska M., Rock K. L., Goldberg A. L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993 Sep 16;365(6443):264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- Gould K. G., Scotney H., Brownlee G. G. Characterization of two distinct major histocompatibility complex class I Kk-restricted T-cell epitopes within the influenza A/PR/8/34 virus hemagglutinin. J Virol. 1991 Oct;65(10):5401–5409. doi: 10.1128/jvi.65.10.5401-5409.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Sasao F., Yamanaka K., Bennink J. R., Yewdell J. W. Recognition of noninfectious influenza virus by class I-restricted murine cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):606–610. [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Kerr L. A., Mockridge I., Elliott T., Bastin J., Uchanska-Ziegler B., Ziegler A., Trowsdale J., Townsend A. Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature. 1992 Feb 13;355(6361):641–644. doi: 10.1038/355641a0. [DOI] [PubMed] [Google Scholar]
- Klar D., Hämmerling G. J. Induction of assembly of MHC class I heavy chains with beta 2microglobulin by interferon-gamma. EMBO J. 1989 Feb;8(2):475–481. doi: 10.1002/j.1460-2075.1989.tb03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassam N., Jay G. Suppression of MHC class I RNA in highly oncogenic cells occurs at the level of transcription initiation. J Immunol. 1989 Dec 1;143(11):3792–3797. [PubMed] [Google Scholar]
- Michalek M. T., Grant E. P., Gramm C., Goldberg A. L., Rock K. L. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993 Jun 10;363(6429):552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Ortiz-Navarrete V., Neefjes J., Goulmy E., van de Wal Y., Spits H., Powis S. J., Butcher G. W., Howard J. C., Walden P. Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature. 1992 Nov 12;360(6400):174–177. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. Genes in the MHC that may affect antigen processing. Curr Opin Immunol. 1992 Feb;4(1):70–73. doi: 10.1016/0952-7915(92)90128-2. [DOI] [PubMed] [Google Scholar]
- Ogata M., Shimizu J., Kosaka H., Maekawa R., Shimizu K., Fujiwara H., Hamaoka T. Expression of H-2 antigens and inducibility of antitumor immune responses in various tumor cell clones established from methylcholanthrene-induced fibrosarcomas. Jpn J Cancer Res. 1986 Nov;77(11):1134–1141. [PubMed] [Google Scholar]
- Rammensee H. G., Falk K., Rötzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- Restifo N. P., Esquivel F., Asher A. L., Stötter H., Barth R. J., Bennink J. R., Mulé J. J., Yewdell J. W., Rosenberg S. A. Defective presentation of endogenous antigens by a murine sarcoma. Implications for the failure of an anti-tumor immune response. J Immunol. 1991 Aug 15;147(4):1453–1459. [PMC free article] [PubMed] [Google Scholar]
- Restifo N. P., Esquivel F., Kawakami Y., Yewdell J. W., Mulé J. J., Rosenberg S. A., Bennink J. R. Identification of human cancers deficient in antigen processing. J Exp Med. 1993 Feb 1;177(2):265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F. M., Cochet M. M., Fellous M. Interferon and major histocompatibility complex genes: a model to analyse eukaryotic gene regulation? Interferon. 1986;7:47–87. [PubMed] [Google Scholar]
- Schumacher T. N., Heemels M. T., Neefjes J. J., Kast W. M., Melief C. J., Ploegh H. L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990 Aug 10;62(3):563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Cerundolo V., Colonna M., Cresswell P., Townsend A., DeMars R. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature. 1992 Feb 13;355(6361):644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Trowsdale J. The transporters associated with antigen presentation. Semin Cell Biol. 1993 Feb;4(1):53–61. doi: 10.1006/scel.1993.1007. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]