Abstract
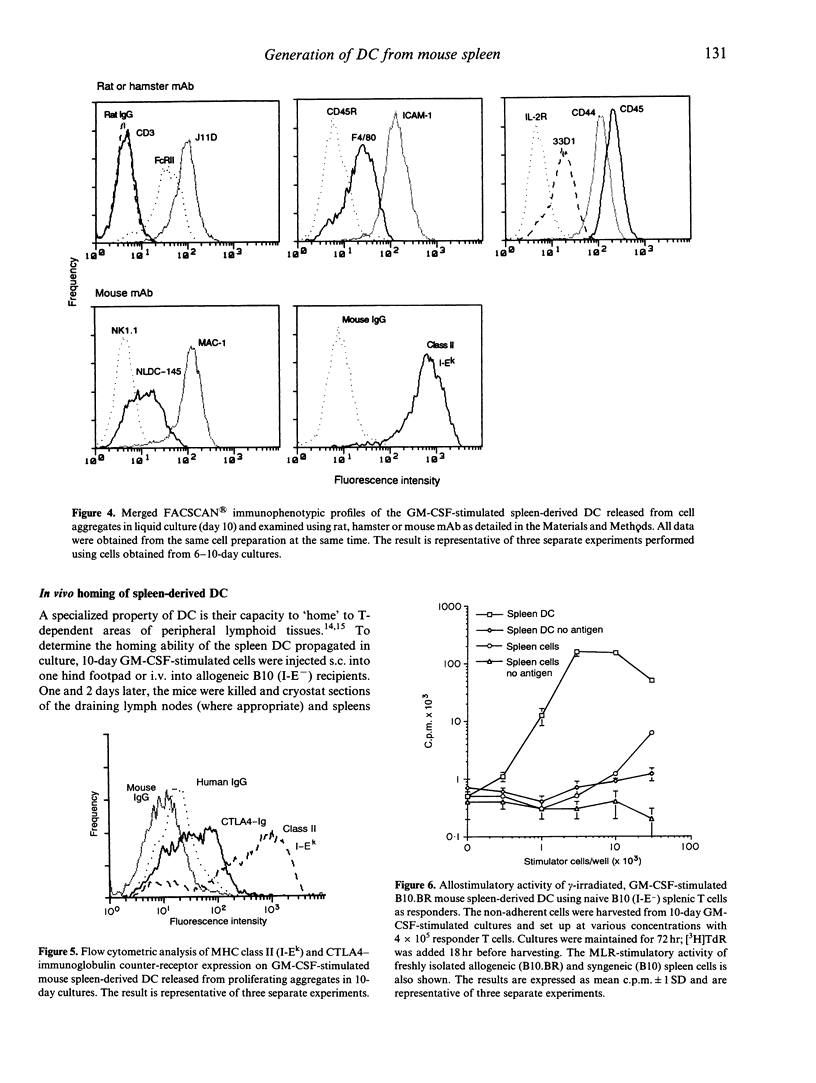
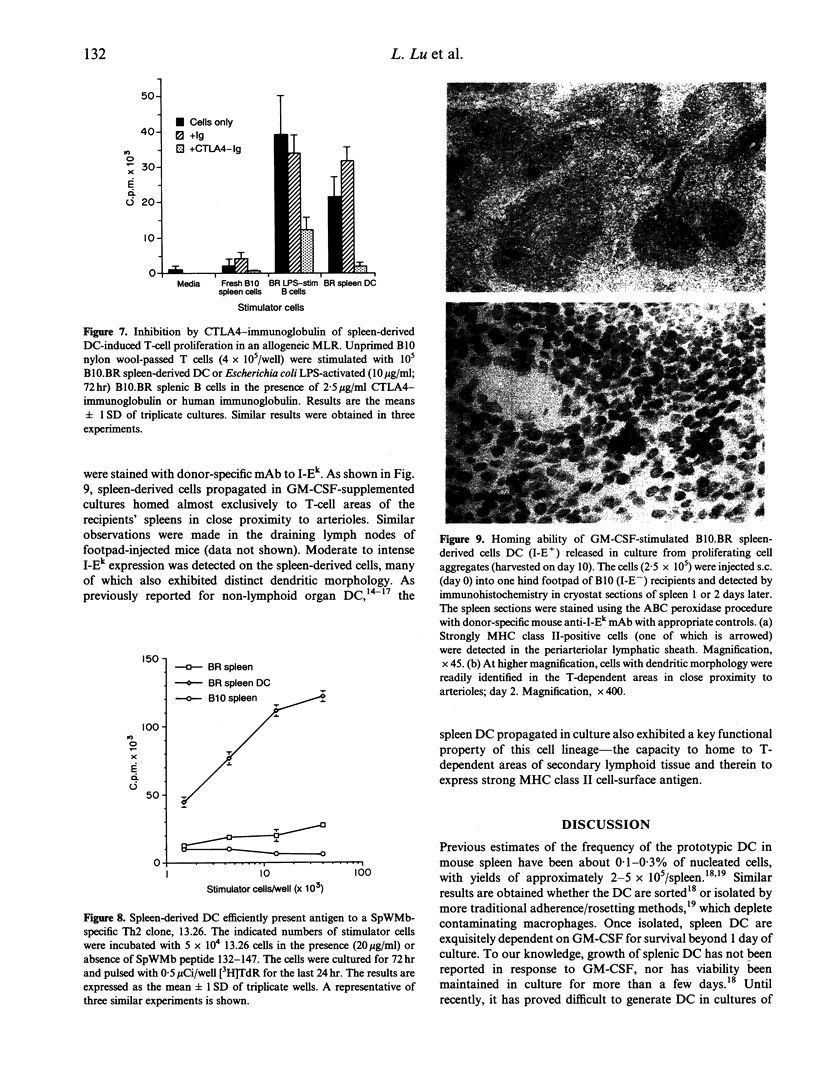
In all tissues that have been studied to date, dendritic leucocytes constitute only a small proportion of total cells and are difficult both to isolate and purify. This study reports on a method for the propagation of large numbers of dendritic cells (DC) from mouse spleen using granulocyte-macrophage colony-stimulating factor (GM-CSF) and their characteristics. Within a few days of liquid culture in GM-CSF, B10 BR (H-2k, I-E+) mouse splenocytes formed loosely adherent myeloid cell clusters. Mononuclear progeny released from these clusters at and beyond 4 days exhibited distinct dendritic morphology and strongly expressed leucocyte common antigen (CD45), CD11b, heat-stable antigen, Pgp-1 (CD44) and intercellular adhesion molecule-1 (ICAM-1; CD54). The intensity of expression of the DC-restricted markers NLDC 145 and 33D1, the macrophage marker F4/80, and Fc gamma RII (CDw32) was low to moderate, whereas the cells were negative for CD3, CD45RA and NK1.1. High and moderate levels, respectively, of cell surface staining for major histocompatibility complex (MHC) class II (I-Ek) and the B7 antigens (counter-receptors of CTLA4, a structural homologue of CD28) were associated with potent stimulation of unprimed, allogeneic T cells (B10; H-2b, I-E-). DC propagated in a similar fashion from DBA/2 mouse spleen proved to be strong antigen-presenting cells (APC) for MHC-restricted, syngeneic T-helper type 2 (Th2) cell clones specifically responsive to sperm whale myoglobin. Footpad or intravenous injection of GM-CSF-stimulated B10.BR spleen-derived DC into B10 (H-2b, I-E-) recipients resulted in homing of the allogeneic cells to T-cell-dependent areas of lymph nodes and spleen, where they strongly expressed donor MHC class II antigen 1-2 days later. These findings indicate that cells can be propagated from fresh splenocyte suspensions that exhibit distinctive features of DC, namely morphology, motility, cell-surface phenotype, potent allogeneic and syngeneic APC function and in vivo homing ability. Propagation of DC in this manner from progenitors present in lymphoid tissue provides an alternative and relatively convenient source of high numbers of these otherwise difficult to isolate but functionally important APC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Kupiec-Weglinski J. W., Hankins D. F., Morris P. J. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988 Feb 1;167(2):646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers W. E., Berkowitz M. R. Differentiation of dendritic cells in cultures of rat bone marrow cells. J Exp Med. 1986 Apr 1;163(4):872–883. doi: 10.1084/jem.163.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M. T., Inaba K., Witmer-Pack M. D., Gezelter S., Steinman R. M. Use of the fluorescence activated cell sorter to enrich dendritic cells from mouse spleen. J Immunol Methods. 1990 Oct 4;133(1):55–66. doi: 10.1016/0022-1759(90)90318-p. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992 Dec 1;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Pack M. W., Aya H., Inaba M., Sudo T., Wolpe S., Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992 May 1;175(5):1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Morris P. J., Austyn J. M. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Steinman R. M., Witmer-Pack M., Hankins D. F., Morris P. J., Austyn J. M. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990 Nov 1;172(5):1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Woo J., Rao A. S., Li Y., Watkins S. C., Qian S., Starzl T. E., Demetris A. J., Thomson A. W. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994 Jun 1;179(6):1823–1834. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J. P., Witmer-Pack M. D., Agger R., Crowley M. T., Lawless D., Steinman R. M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990 May 1;171(5):1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P. A., Livingstone A. M., Fathman C. G. Correlation of T cell receptor V beta gene family with MHC restriction. J Exp Med. 1987 Aug 1;166(2):583–588. doi: 10.1084/jem.166.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. D., Fryer P. R., Clifford C., Kirk A., Tikerpae J., Knight S. C. Identification of hematopoietic progenitors of macrophages and dendritic Langerhans cells (DL-CFU) in human bone marrow and peripheral blood. Blood. 1990 Sep 15;76(6):1139–1149. [PubMed] [Google Scholar]
- Reid C. D., Stackpoole A., Meager A., Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992 Oct 15;149(8):2681–2688. [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994 Apr 1;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974 Feb 1;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Woo J., Lu L., Rao A. S., Li Y., Subbotin V., Starzl T. E., Thomson A. W. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation. 1994 Aug 27;58(4):484–491. doi: 10.1097/00007890-199408270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]