Abstract
Eukaryotic plasma membranes generally display asymmetric lipid distributions with the aminophospholipids concentrated in the cytosolic leaflet. This arrangement is maintained by aminophospholipid translocases (APLTs) that use ATP hydrolysis to flip phosphatidylserine (PS) and phosphatidylethanolamine (PE) from the external to the cytosolic leaflet. The identity of APLTs has not been established, but prime candidates are members of the P4 subfamily of P-type ATPases. Removal of P4 ATPases Dnf1p and Dnf2p from budding yeast abolishes inward translocation of 6-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)aminocaproyl] (NBD)-labeled PS, PE, and phosphatidylcholine (PC) across the plasma membrane and causes cell surface exposure of endogenous PE. Here, we show that yeast post-Golgi secretory vesicles (SVs) contain a translocase activity that flips NBD-PS, NBD-PE, and NBD-PC to the cytosolic leaflet. This activity is independent of Dnf1p and Dnf2p but requires two other P4 ATPases, Drs2p and Dnf3p, that reside primarily in the trans-Golgi network. Moreover, SVs have an asymmetric PE arrangement that is lost upon removal of Drs2p and Dnf3p. Our results indicate that aminophospholipid asymmetry is created when membrane flows through the Golgi and that P4-ATPases are essential for this process.
INTRODUCTION
Because of the polar character of the lipid head group, spontaneous transbilayer movement of phospholipids is slow (Kornberg and McConnell, 1971; Cribier et al., 1993). As a consequence of this restricted motion, the two leaflets of a membrane can be completely different in phospholipid composition. A prominent example is the plasma membrane of animal cells where phospholipids display an asymmetric distribution with phosphatidylcholine (PC) and sphingomyelin (SM) concentrated in the exoplasmic leaflet and phosphatidylserine (PS) and phosphatidylethanolamine (PE) restricted to the cytosolic leaflet (Pomorski et al., 2004). Phospholipid synthesis occurs primarily on the cytosolic leaflet of the endoplasmic reticulum (ER) (Bell et al., 1981). In this organelle, one-half of the newly synthesized phospholipids must be flipped to the other side to produce a bilayer. The ER membrane contains one or more undefined proteins, termed flippases, which mediate a fast, energy-independent flip-flop of most lipid classes in both directions (Bishop and Bell, 1985; Pomorski et al., 2004). As facilitators of nonvectorial lipid transport, ER flippases would promote a random or symmetrical lipid distribution across the bilayer. This would imply that asymmetry is created as membrane flows through the Golgi or on arrival at the plasma membrane.
Lipid asymmetry cannot be fully explained by sidedness of lipid synthesis or breakdown and is thought to rely, at least in part, on energy-dependent flippases or translocases that use ATP hydrolysis to move lipids against a concentration gradient. The best-studied example is the aminophospholipid translocase (APLT), which catalyzes a fast inward movement of PS and PE across the plasma membrane (the term aminophospholipid herein refers exclusively to the primary amine containing phospholipids PE and PS). Although first discovered in human erythrocytes (Seigneuret and Devaux, 1984), APLT activities have now been detected in the plasma membranes of many nucleated cells (Pomorski et al., 2004) as well as in bovine chromaffin granules (Zachowski et al., 1989) and the trans-Golgi network (TGN) of budding yeast (Natarajan et al., 2004). Even though the identity of the APLT remains to be established, strong candidates are members of the P4 subfamily of P-type ATPases (herein referred to as P4 ATPases). The first member of this family was identified upon purification of an APLT activity from bovine chromaffin granules (Tang et al., 1996). This protein, also known as ATPase II, displays a striking similarity to the yeast protein Drs2p. The observation that removal of Drs2p caused a specific defect in the inward translocation of 6-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)aminocaproyl] (NBD)-labeled PS across the yeast plasma membrane provided further support for a role of P4 ATPases in aminophospholipid transport (Tang et al., 1996; Gomes et al., 2000). However, other studies reported no dependency on Drs2p for aminophospholipid transport (Siegmund et al., 1998; Marx et al., 1999). In addition, Drs2p was found to localize to the TGN rather than the plasma membrane (Chen et al., 1999), indicating that this protein unlikely contributes directly to the APLT activity in the plasma membrane. Analogous to clathrin mutants in yeast, cells lacking Drs2p display significant defects in protein transport from the TGN (Chen et al., 1999; Gall et al., 2002; Hua et al., 2002), which could perturb delivery of the APLT to the plasma membrane.
Recent findings have reinforced the idea that P4 ATPases directly participate in aminophospholipid transport. The yeast P4 ATPase family comprises five members with overlapping in vivo functions, namely, Drs2p, Dnf1p, Dnf2p, Dnf3p, and Neo1p (Hua et al., 2002). Dnf1p and Dnf2p localize primarily to the plasma membrane, and loss of these two proteins disrupts the inward translocation of NBD-PS, NBD-PE, and NBD-PC across the plasma membrane and causes an increased exposure of endogenous PE at the cell surface (Pomorski et al., 2003). Moreover, TGN membranes purified from strains that lack the Dnf proteins and contain a temperature-sensitive form of Drs2p become defective in NBD-PS translocation from the luminal to the cytosolic leaflet when shifted to the nonpermissive temperature (Natarajan et al., 2004). The latter finding provides the strongest evidence to date that P4 ATPases serve a primary role in lipid transport. The specificity of Drs2p for NBD-PS suggested that translocation of PS would be required for the function of Drs2p in protein transport from the TGN. However, PS-deficient yeast strains transport proteins normally via the secretory pathway and still require Drs2p to produce a specific class of secretory vesicles (Natarajan et al., 2004). This finding indicated that PS is not an obligatory substrate for the in vivo function of Drs2p, suggesting that Drs2p translocates another substrate with a critical role in protein transport from the TGN. Dnf3p colocalizes with Drs2p in the TGN (Hua et al., 2002), whereas Neo1p is primarily associated with the endosomal system (Wicky et al., 2004). Whether these P4 ATPases help establish lipid asymmetry in membranes en route to the cell surface is not known.
To further investigate the origin of lipid asymmetry, we analyzed the transbilayer movement and arrangement of phospholipids in post-Golgi secretory vesicles isolated from the late secretory yeast mutant sec6-4. We find that these vesicles contain an ATP-dependent translocase activity that flips NBD labeled PS, PE and PC from the luminal to the cytosolic leaflet. This activity is independent of the plasma membrane P4 ATPases Dnf1p and Dnf2p, but instead requires Drs2p and Dnf3p. Trinitrobenzene sulfonic acid labeling shows that post-Golgi secretory vesicles have an asymmetric PE distribution with the bulk of PE located in the cytosolic leaflet, and that this asymmetry is strictly dependent on Drs2p and Dnf3p. These findings indicate that P4 ATPases Drs2p and Dnf3p help create aminophospholipid asymmetry in membranes en route to the cell surface, presumably at the level of the Golgi.
MATERIALS AND METHODS
Strains and Plasmids
Yeast strains were routinely grown in synthetic dextrose (SD) medium at 27°C. Gene deletion phenotypes were characterized in strain EHY227 (MATα sec6-4 TPI1::SUC2::TRP1 ura3-52 his3-Δ200 leu2-3-112 trp1-1). For deletion of DRS2, DNF1, DNF2, and DNF3, 450- to 550-base pair fragments of the promotor and open reading frame 3′ end of each gene were amplified by polymerase chain reaction (PCR) from yeast genomic DNA and cloned on either site of a loxP-HIS3-loxP cassette in a pBluescript KS– vector, as described previously (Pomorski et al., 2003). The deletion constructs were linearized with NotI and MluI and then transformed into EHY227, yielding sec6-4Δdrs2 (TPY007), sec6-4Δdnf1 (TPY044), and sec6-4Δdnf3 (TPY123). Multiple deletions were performed sequentially in EHY227 by repeated use of the loxP-HIS3-loxP cassette and subsequent removal of the HIS3 marker by excisive recombination using Cre recombinase, yielding sec6-4Δdnf1Δdnf2 (TPY047), sec6-4Δdrs2Δdnf3 (TPY125) and sec6-4Δdrs2Δdnf1Δdnf2Δdnf3 (TPY127). In each case, the correct integration or excision event was confirmed by PCR. In the sec6-4 and sec6-4Δdrs2Δdnf3 strains, endogenous Pma1p was tagged at its amino terminus with one copy of the hemagglutinin (HA) epitope using integration plasmid pRS305Δ51 (Ziman et al., 1996), yielding sec6-4 PMA1-HA (TPY149), sec6-4Δdrs2Δdnf3 PMA1-HA (TPY143) and sec6-4Δdrs2Δdnf1Δdnf2Δdnf3 PMA1-HA (TPY147). The open reading frame of DRS2 was cloned into pRS426 (2μ, URA3) behind the PMA1 promotor using the gap-repair method.
Preparation and Fractionation of Secretory Vesicle (SV)-enriched Membranes
Sec6-4 strains were inoculated into 1 liter of SD medium and then grown for 14–16 h at 27°C to 0.5 optical density (OD)600/ml. One-half of the culture was shifted to 38°C for 90 min to allow accumulation of SVs, and the other half was grown at 27°C. Cells used for the membrane fractionation experiments shown in Figures 1 and 5 were spheroplasted and then lysed in lysis buffer using a Dounce homogenizer as described previously (Harsay and Schekman, 2002). Cells used for lipid translocation and trinitrobenzene sulfonic acid (TNBS) labeling experiments (all remaining figures) were lysed by vortexing 20 times for 30 s with glass beads (425–600 μm) in ice-cold lysis buffer (0.8 M sorbitol, 10 mM trieethanolamine, 1 mM EGTA, and protease inhibitors, pH 7.2). Lysates were subjected to differential centrifugation (500 × gav for 10 min; 13,000 × gav for 20 min) to remove most of the nuclei, vacuoles, mitochondria, and plasma membrane. SV-enriched membranes were collected by high-speed centrifugation (100,000 × gav; 90 min; 4°C) on a 60% (wt/wt) sucrose cushion. For lipid translocation studies, membranes were resuspended in K-glutamate buffer (120 mM potassium glutamate, 15 mM KCl, 5 mM NaCl, 3 mM MgCl2, 2 mM EGTA, and 20 mM HEPES-KOH, pH 7.2) containing 20% glycerol and stored at –80°C. For fractionation studies, membranes were collected on a 60% (wt/wt) Nycodenz cushion, resuspended in 1.5 ml of lysis buffer, adjusted to 30% (wt/wt) Nycodenz, and then loaded at the bottom of an 11-ml linear 12–22% (wt/wt) Nycodenz/0.8 M sorbitol gradient. After centrifugation at 100,000 × gav for 16 h at 4°C in a Beckman SW41Ti rotor, 20 × 0.6-ml fractions were collected from the top. Equal volumes per fraction were subjected to immunoblotting and analyzed for ATPase, invertase, and Kex2p enzyme activities as described previously (Harsay and Bretscher, 1995; Cunningham and Wickner, 1989). Immunoblots were probed with a monoclonal antibody (mAb) against Dpm1p (Molecular Probes, Eugene, OR), a polyclonal antibody against the HA epitope (Santa Cruz Biotechnology, Santa Cruz, CA), and an affinity-purified polyclonal antibody against Drs2p (provided by Todd Graham, Vanderbilt University, Nashville, TN). Horseradish peroxidase-conjugated secondary antibodies were from Bio-Rad (Hercules, CA). Blots were developed using enhance chemiluminescence (Pierce Chemical, Rockford, IL).
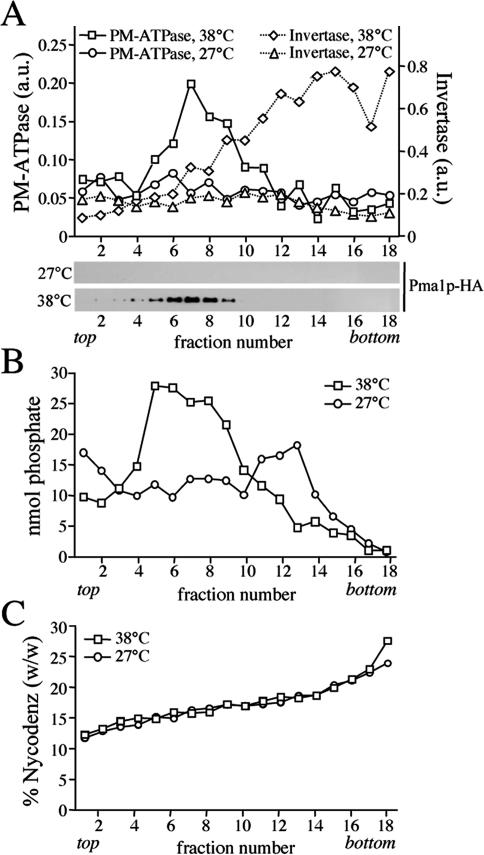
Figure 1.
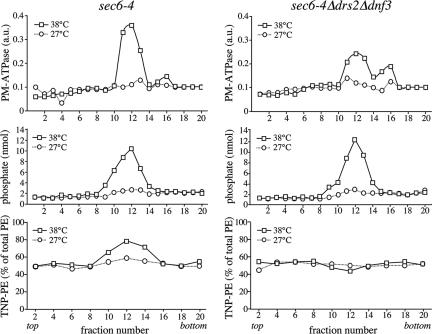
Gradient fractionation of post-Golgi SVs accumulated in 38°C-shifted sec6-4 cells. Membranes enriched in SVs were prepared from 38°C-shifted or 27°C-grown sec6-4 cells by differential centrifugation, loaded on the bottom of a linear Nycodenz/sorbitol gradient, and then floated to equilibrium by centrifugation. (A) Fractions were collected from the top and analyzed for enzyme activities and by immunoblotting. Enzyme activities were determined as described in Materials and Methods and expressed in arbitrary units (a.u.) based on the absorbance measured at 660 nm (PM-ATPase) or 540 nm (invertase). Immunoblots were probed with a mAb against the HA epitope to detect HA-tagged Pma1p. (B) Lipid phosphorus content per fraction was determined as described in Materials and Methods. (C) Fraction densities were measured by reading refractive indices on a Bausch & Lomb refractometer.
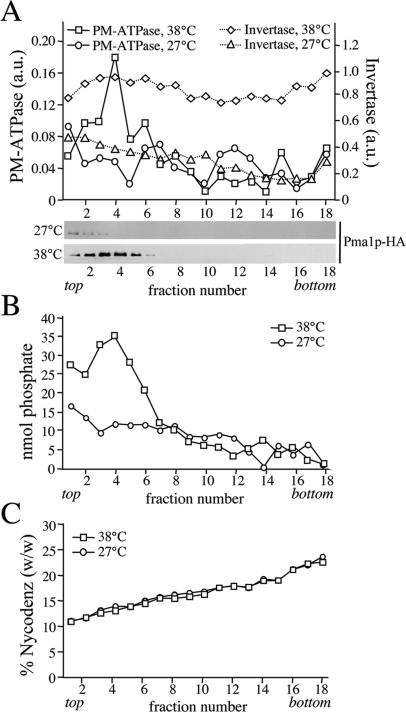
Figure 5.
Formation of Pma1p-transporting SVs is independent of Drsp2 and Dnf3p. SV-enriched membranes were prepared from 38°C-shifted or 27°C-grown sec6-4Δdrs2Δdnf3 cells by differential centrifugation, loaded on the bottom of a linear Nycodenz/sorbitol gradient and then floated to equilibrium by centrifugation. (A) Fractions were collected from the top and analyzed for enzyme activities and by immunoblotting as in the legend to Figure 1. (B) Lipid phosphorus content was determined as described in Materials and Methods. (C) Fraction densities were measured by reading refractive indices on a Bausch & Lomb refractometer.
Immuno-isolation of SVs
Immuno-isolations of Pma1p-HA–containing SVs were performed using magnetic Dyna beads protein G (Dynal Biotech, Hamburg, Germany) loaded with mouse monoclonal anti-HA antibody 12CA5 (Roche Diagnostics, Mannheim, Germany; 0.36 μg of antibody/μl of bead-slurry). Per immuno-isolation, a 500-μl reaction was prepared in lysis buffer containing 200 μl of 12CA5-bound Dyna bead slurry, 5 mg/ml bovine serum albumin (BSA), and 100 μl of Nycodenz gradient PM-ATPase peak fractions (6–8) obtained by fractionation of SV-enriched membranes from 1 g of 38°C-shifted sec6-4 cells. As control for the specificity of the immuno-isolation procedure, one reaction was set up using 12CA5-bound beads preincubated at 4°C for 1 h with 200 μg of synthetic peptide corresponding to the HA epitope (YPYDVPDYA). The reactions were rotated gently at 4°C for 2 h. Beads were separated from the supernatants and washed twice over 2 h in lysis buffer containing 5 mg/ml BSA and once over 30 min in lysis buffer. Membranes in supernatants were collected by high-speed centrifugation. Beads and membrane pellets were each resuspended in 100 μl of SDS-PAGE sample buffer and analyzed by immunoblotting.
NBD-Lipid Translocation Assay
Palmitoyl-(NBD-hexanoyl)-PS (NBD-PS), palmitoyl-(NBD-hexanoyl)-PE (NBD-PE), palmitoyl-(NBD-hexanoyl)-PC (NBD-PC), and (NBD-hexanoyl)-sphingosine-1-phosphocholine (NBD-sphingomyelin; NBD-SM) were from Avanti Polar Lipids (Birmingham, AL). Except for NBD-PE, lipid analogues were resuspended to a final concentration of 100 μM in K-glutamate buffer. For NBD-PE, a 1 mM stock was prepared in dimethyl sulfoxide. SV-enriched membranes were diluted to 1.5 μmol of phospholipid/ml in K-glutamate buffer containing an ATP-regenerating system (10 mM phosphocreatine; Sigma-Aldrich, St. Louis, MO; 25 U/ml creatine phosphokinase; Calbiochem, Darmstadt, Germany). Translocation assays were set up in glass tubes at 25°C by mixing 300 μl of membrane suspension with NBD-lipid suspension (3 μl for NBD-PE; 30 μl for all other NBD-lipids; corresponded to ∼0.6 mol% of total phospholipid content). The degree of NBD-lipid insertion was assessed by monitoring the time course of fluorescence as described in Supplemental Figure 1. Except for NBD-PE, label insertion was complete within 1 min. With NBD-PE, longer incubation time was required (10 min at 25°C). After 3 h of incubation, reactions were split, 1 mM ATP (Roche Diagnostics, Indianapolis, IN) was added to one-half, and the incubation was continued an additional 2 h. Thin layer chromatography (TLC) analysis revealed that the majority (>85%) of NBD-PC, NBD-PE, and NBD-SM remained intact throughout the incubation. Addition of ATP or the removal of P4 ATPases had no effect on the level of lipid hydrolysis. Hydroxylamine (5 mM) was added to reactions with NBD-PS to block its conversion to NBD-PE (Natarajan et al., 2004). At hourly time points, two 10-μl aliquots were removed and mixed with 90 μl of ice-cold K-glutamate buffer and 90 μl of 4% (wt/vol) fatty acid-free BSA (Sigma-Aldrich)-containing K-glutamate buffer. After 10 min on ice, reactions were diluted with 100 μl of ice-cold K-glutamate buffer, and fluorescence intensity was recorded at 540 nm (excitation, 470 nm; slit width, 4 nm; and resolution, 1 s) before and after addition of 0.5% Triton X-100. Fluorescence of each sample was measured at 25°C using quartz microcuvettes and an Aminco Bowman Series 2 spectrofluorometer (SLM Instruments, Rochester, NY). The accessible pool (Pext) of fluorescent lipid was calculated as Pext = [1 – (FBSA/Fbuffer)]/[1 – 0.55] × 100, where FBSA is the fluorescence (normalized to fluorescence after addition of Triton X-100) of membranes after extraction with BSA, Fbuffer is the fluorescence (normalized to fluorescence after addition of Triton X-100) of membranes in buffer without BSA, Pext is the proportion of labeled lipid that is extracted by BSA, and the relative quantum yield of BSA-bound fluorescent lipid is 0.55 compared with a value of 1 for membrane-associated fluorescent lipid (Kubelt et al., 2002). The reduced quantum yield of BSA-bound versus membrane-associated NBD-lipid was similar for all four analogues tested. The back-exchange procedure was sufficient to extract >90% of membrane-associated NBD-lipid at time 0 (10 min after probe addition). This amount was set to 100% for kinetic measurements.
TNBS Labeling
Sec6-4 strains were grown overnight in SD medium at 27°C to 0.7 OD600/ml. One-half of the culture was shifted to 38°C for 90 min, and the other half grown at 27°C. Cells were lysed by vortexing with glass beads in KHCO3 buffer (120 mM KHCO3, 15 mM KCl, 5 mM NaCl, 0.8 mM CaCl2, 2 mM MgCl2, 1.6 mM EGTA, and protease inhibitors, pH 8.5). Lysates were spun at 500 × gav for 10 min at 4°C. The postnuclear supernatant was mixed with an equal volume of KHCO3 buffer containing 10 mM TNBS (Sigma-Aldrich) or 10 mM 1-fluoro-2,4-dinitrobenzene (FDNB; Sigma-Aldrich) and incubated at 20°C with periodic mixing. Control incubations were subjected to sonication using a Sonifier 250 (duty cycle 20%, output control 2; Branson, Danbury, CT) for 3 min on ice to determine efficiency of labeling and quenching. For analysis of TNBS labeling on SV-enriched membranes, samples were transferred at given time points on ice, and 3 volumes of stop buffer (0.93 M sorbitol, 500 mM glycylglycine, and 100 mM citric acid, pH 5.0) was added. SV-enriched membranes were collected by differential centrifugation and then subjected to lipid analysis (see below). For fractionation of TNBS-labeled SVs on Nycodenz gradients, postnuclear supernatants were incubated with TNBS for 45 min at 20°C and then centrifuged at 13,000 × gav for 20 min at 4°C. The supernatant was mixed with 3 volumes of ice-cold stop buffer, and SVs were collected by centrifugation at 100,000 × gav for 90 min at 4°C on a 60% (wt/wt) Nycodenz cushion. SVs were resuspended in 1.5 ml of stop buffer and then fractionated on a Nycodenz gradient as described above. Equal volumes per fraction were used for Western blot analysis, total phosphate determination, and lipid analysis (as described in Pomorski et al., 2003).
RESULTS
Yeast Post-Golgi SVs Contain Phospholipid Translocase Activity
Bovine brain chromaffin granules contain an APLT activity thought to create lipid asymmetry across the bilayer (Zachowski et al., 1989). To investigate whether a similar activity is present in yeast post-Golgi SVs, a high-speed membrane pellet enriched for SVs was isolated from the late secretory mutant sec6-4 and tested for ATP-dependent NBD-phospholipid transport activity. Sec6-4 cells grow like wild-type cells at 27°C, but growth ceases at 38°C, and the cells accumulate two populations of 100-nm SVs that can be separated by equilibrium isodensity centrifugation on Nycodenz gradients, namely, a light density population containing the major plasma membrane ATPase, Pma1p, and a higher density population containing the periplasmic enzyme invertase (Harsay and Schekman, 2002). SV-enriched membranes were prepared by differential centrifugation of total cell lysates derived from 38°C-shifted sec6-4 cells as described in Materials and Methods. Fractionation on a linear Nycodenz gradient revealed two peaks of enzyme activities that were absent when fractionation was performed on membranes from 27°C-grown cells: a low-density peak containing ATPase activity and a higher density peak containing invertase activity (Figure 1A). Western blot analysis showed that Pma1p cofractionates with the lower density membranes, confirming that the detected ATPase activity is because of this protein. Using a phosphorus assay, a peak comprising the bulk of phospholipids (>80% of total) was found to coincide with the ATPase peak in gradients from 38°C-shifted cells (Figure 1B). This peak was absent in gradients from 27°C-grown cells. A comparison of the phospholipid content between membrane pellets prepared from equal amounts of 38°C-shifted and 27°C-grown cells indicated that ∼65% of the phospholipids in the 38°C pellet is derived from SVs. Based on the phospholipid fractionation profiles, we estimate that the Pma1p class of SVs in 38°C membrane pellets outnumber the invertase class at least five times. This was confirmed by counting vesicular profiles in electronmicrographs of negatively stained membranes derived from Pma1p and invertase peak fractions (our unpublished data).
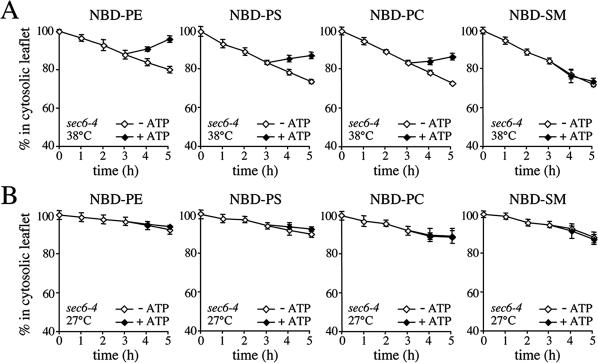
SV-enriched membranes from 38°C-shifted sec6-4 cells were next analyzed for ATP-dependent NBD phospholipid transport activity using a BSA back-extraction assay (Zachowski et al., 1989; Natarajan et al., 2004). To this end, membranes were labeled with NBD phospholipids and incubated at 25°C in the presence or absence of ATP. At hourly time points, two aliquots of each sample were taken and mixed with buffer containing fatty acid-free BSA to extract NBD phospholipid from the cytosolic leaflet or buffer without BSA. Extraction of NBD-lipids by BSA causes a decrease in the total fluorescence of the sample because NBD-lipid bound to BSA has a 1.8-fold lower quantum yield than membrane-bound NBD-lipid (Kubelt et al., 2002). Thus, the amount of BSA-extractable probe can be determined without physical separation of vesicles from BSA by measuring the reduction in NBD fluorescence emission. The extent of fluorescence reduction can then be used to calculate the percentage of NBD phospholipid in the cytosolic leaflet (see Materials and Methods for further details). NBD-PE incubated with SV-enriched membranes in the absence of ATP gradually moved from the cytosolic to the luminal leaflet, reaching an 80:20 distribution in 5 h (Figure 2A, left). To determine whether these membranes contain an ATP-dependent transport activity capable of translocating NBD-PE back to the cytosolic leaflet, they were preincubated for 3 h in the absence of ATP to allow movement of the lipid to the luminal leaflet until 87% remained in the cytosolic leaflet. ATP was then added and the membranes were incubated for another 2 h. On addition of ATP, a substantial portion of the NBD-PE was transported back to the cytosolic leaflet so that 97% was present in the cytosolic leaflet by the 5-h time point (Figure 2A, left). A similar although less pronounced ATP-dependent redistribution of lipid was observed when membranes were incubated with NBD-PS or NBD-PC, suggesting the presence of a translocase activity that moves PE, PS, and PC analogues from the luminal to the cytosolic leaflet. This activity seemed specific for a subset of phospholipids because the transbilayer movement of NBD-labeled SM, phosphatidic acid, or phosphatidylglycerol was unaffected by ATP (Figure 2A, right; our unpublished data). The translocase activity cofractionated with the Pma1p-containing vesicles on Nycodenz gradients and could not be detected in membranes isolated from 27°C-grown sec6-4 cells (Figure 2B; our unpublished data). Together, these findings indicate that yeast post-Golgi SVs contain one or more phospholipid translocases that specifically transport PE, PS, and PC analogues from the luminal to the cytosolic leaflet.
Figure 2.
SVs accumulated in 38°C-shifted sec6-4 cells contain NBD-phospholipid translocase activity. (A) Membranes enriched in SVs were prepared from 38°C-shifted sec6-4 cells by differential centrifugation, labeled with NBD-lipids, and incubated for 3 h at 25°C without ATP. The reaction was then split, ATP was added to one-half, and the incubation was continued an additional 2 h. At times indicated, the fraction of fluorescent lipid remaining in the cytosolic leaflet was determined by back-extraction using defatted BSA as described in Materials and Methods. Data shown are means ± SD of three independent experiments. (B) SV-enriched membranes prepared from 27°C-grown sec6-4 cells were analyzed for ATP-dependent NBD-lipid transport by BSA back-extraction as in A. Data shown are means ± SD of two independent experiments. Note that the membranes from 27°C-grown cells display an apparent reduction in the passive movement of all four NBD-lipids compared with membranes from 38°C-shifted cells. Given the assay conditions used, this may be explained by an overall increase in the rate of flip-flop because of a larger fraction of ER membranes in the 27°C membrane preparation (also see Figure 6A).
Loss of Drs2p and Dnf3p Abolishes SV-associated Phospholipid Translocase Activity
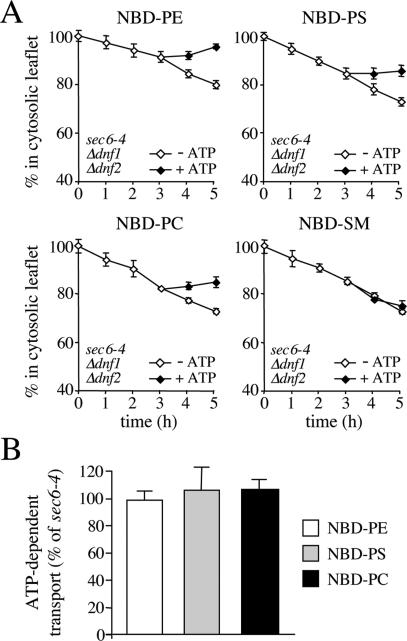
The phospholipid translocase activity detected in yeast SVs differs from the APLT found in bovine chromaffin granules in that it mediates transport of both primary aminophospholipid and PC analogues. The yeast plasma membrane contains two P4 ATPases, Dnf1p and Dnf2p, which are required for inward translocation of NBD-labeled PE, PS, and PC across the plasma membrane (Pomorski et al., 2003). To investigate whether these proteins are also required for the translocase activity associated with yeast SVs, we next assayed SV-enriched membranes from 38°C-shifted sec6-4Δdnf1Δdnf2 cells for ATP-dependent lipid transport activity. Removal of Dnf1p and Dnf2p had no significant effect on the ATP-dependent movement of the PE, PS, and PC analogues (Figure 3).
Figure 3.
SV-associated phospholipid translocase activity is independent of Dnf1p and Dnf2p. (A) SV-enriched membranes were prepared from 38°C-shifted sec6-4Δdnf1Δdnf2 cells, labeled with NBD-lipids, and incubated for 3 h at 25°C without ATP. The reaction was then split, ATP was added to one-half, and the incubation was continued an additional 2 h. At times indicated, the fraction of fluorescent lipid remaining in the cytosolic leaflet was determined as in the legend to Figure 2. (B) As described in A, except that membranes were labeled with NBD-lipid and then incubated for 4 h in the continuous presence or absence of ATP. The level of ATP-dependent lipid transport was calculated by subtracting the accessible pool of fluorescent lipid in membranes incubated without ATP from that in ATP-incubated membranes and expressed as percentage of transport measured in membranes from 38°C-shifted sec6-4 cells. Data shown are means ± SD of at least two independent experiments.
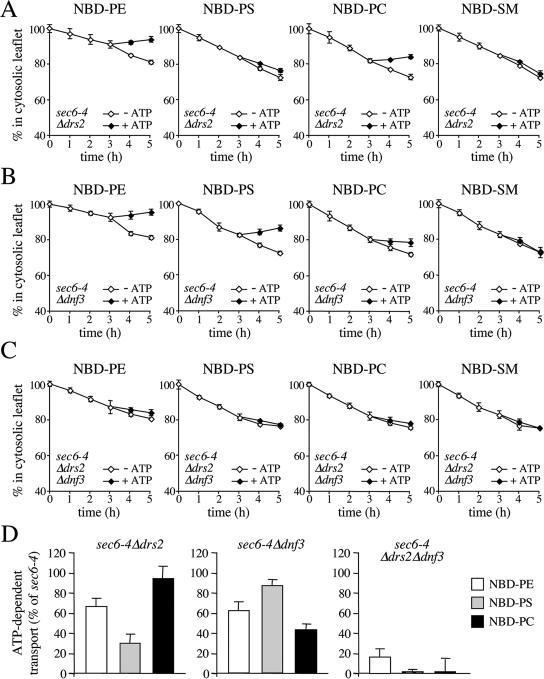
Two other P4 ATPases, Drs2p and Dnf3p, reside primarily in the yeast TGN (Chen et al., 1999; Hua et al., 2002). A recent study showed that ATP hydrolysis by Drs2p is tightly coupled with NBD-PS translocation from the luminal to the cytosolic leaflet of the TGN (Natarajan et al., 2004). When endocytosis is blocked, Drs2p accumulates in the plasma membrane (Saito et al., 2004), suggesting that this protein cycles between the TGN and the plasma membrane. This led us to investigate whether the SV-associated translocase activity is dependent on Drs2p and/or Dnf3p. Remarkably, removal of Drs2p caused a strong reduction in NBD-PS transport without significantly affecting NBD-PC transport (Figure 4, A and D), whereas removal of Dnf3p essentially produced the opposite effect (Figure 4, B and D). Removal of Drs2p or Dnf3p in each case caused a small but significant reduction in NBD-PE transport (Figure 4D). Loss of both P4 ATPases essentially abolished ATP-dependent transport of all three lipid analogues (Figure 4, C and D).
Figure 4.
Loss of Drs2p and Dnf3p abolishes SV-associated phospholipid translocase activity. SV-enriched membranes prepared from 38°C-shifted sec6-4Δdrs2 (A), sec6-4Δdnf3 (B) and sec6-4Δdrs2Δdnf3 cells (C) were labeled with NBD-lipids and incubated for 3 h at 25°C without ATP. The reaction was then split, ATP was added to one-half, and the incubation was continued an additional 2 h. At times indicated, the fraction of fluorescent lipid remaining in the cytosolic leaflet was determined as in the legend to Figure 2. (D) SV-enriched membranes from sec6-4Δdrs2, sec6-4Δdnf3, and sec6-4Δdrs2Δdnf3 cells were labeled with NBD-lipid and then incubated for 4 h in the continuous presence or absence of ATP. The level of ATP-dependent lipid transport was calculated by subtracting the accessible pool of fluorescent lipid in membranes incubated without ATP from that in ATP-incubated membranes and expressed as percentage of transport measured in membranes from 38°C-shifted sec6-4 cells. Data shown are means ± SD of at least two independent experiments.
Drs2p has a critical role in the formation of the high-density, invertase-containing class of SVs (Chen et al., 1999; Gall et al., 2002). To exclude the possibility that the loss of phospholipid translocase activity is because of a general defect in SV formation, membranes derived from 38°C-shifted sec6-4Δdrs2Δdnf3 cells were further analyzed by fractionation on a Nycodenz gradient. As shown in Figure 5, these membranes contained elevated levels of invertase activity in comparison with membranes from 27°C-grown cells, but the invertase activity was now evenly spread throughout the gradient. This result is consistent with the previous finding that loss of Drs2p perturbs formation of the high-density class of SVs, which may cause accumulation of invertase in the light vesicles, Golgi, and/or endosomal compartments. Plasma membrane ATPase activity, Pma1p, and the bulk of phospholipids, on the other hand, cofractionated in a single low-density peak that was absent when fractionation was performed on membranes from 27°C-grown cells (Figure 5). A comparison of the phospholipid content between membranes isolated from equal amounts of 38°C-shifted and 27°C-grown sec6-4 or sec6-4Δdrs2Δdnf3 cells showed that in each case, ∼65% of the phospholipids in the 38°C pellet is derived from SVs (our unpublished data). These results demonstrate that formation of the low-density, Pma1p-containing SVs is essentially independent of Drs2p and Dnf3p.
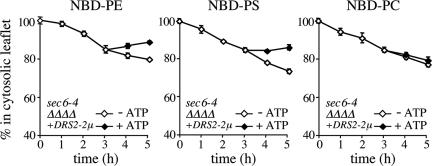
Cells lacking Drs2p show significant defects in protein transport from the TGN and a reduction in the amount of Dnf1p at the cell surface (Chen et al., 1999; Hua et al., 2002). To exclude the possibility that the lipid translocation defect in sec6-4Δdrs2Δdnf3-derived SVs is primarily because of a loss of Dnf1p and Dnf2p from these vesicles, we created a sec6-4Δdrs2Δdnf1,2,3 mutant strain expressing Drs2p from a multicopy plasmid. As shown in Figure 6, SV-enriched membranes derived from this strain contain a translocase activity recognizing NBD-labeled PS and PE, but not NBD-PC. This result suggests that Drs2p plays a direct and specific role in the translocation of phospholipid analogues from the luminal to the cytosolic leaflet of Pma1p-containing SVs.
Figure 6.
SV-associated translocase activity from a Dnf1,2,3-deficient mutant recognizes NBD-labeled PS and PE but not NBD-PC. SV-enriched membranes prepared from 38°C-shifted sec6-4Δdrs2Δdnf1,2,3 mutant cells expressing Drs2p from a multicopy (2μ) plasmid were labeled with NBD-lipids and incubated for 3 h at 25°C without ATP. The reaction was then split, ATP was added to one-half, and the incubation was continued an additional 2 h. At times indicated, the fraction of fluorescent lipid remaining in the cytosolic leaflet was determined as in the legend to Figure 2. Data shown are means ± SD of two independent experiments.
Drs2p Is Incorporated into the Low-Density Class of SVs
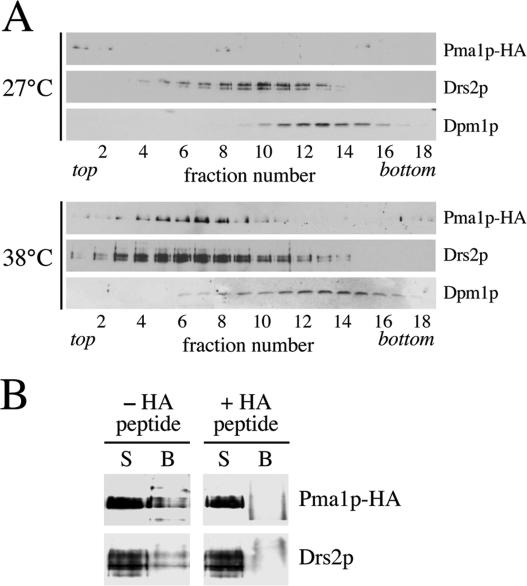
The above-mentioned findings imply that Drs2p (and Dnf3p) are copackaged with Pma1p in the low-density class of SVs. To test this prediction, we analyzed the fractionation profile of Drs2p in the Nycodenz gradients shown in Figure 1. In the gradient derived from 27°C-grown sec6-4 cells, Drs2p peaked in fraction 10 (Figure 7A). However, in the gradient from 38°C-shifted cells, the bulk of Drs2p migrated to a lower density region containing the peak of Pma1p-positive vesicles. Moreover, whereas both gradients contained similar amounts of the ER marker Dpm1p, the 38°C gradient contained ∼4 times more Drs2p than the 27°C gradient (Figure 7A). These results are consistent with incorporation of Drs2p into the low-density class of SVs but could also be ascribed to a temperature-induced change in the fractionation profile of the TGN. To distinguish between these possibilities, we compared the fractionation behavior of Drs2p with that of Kex2p, a TGN-resident enzyme that does not traverse the plasma membrane and hence should not get incorporated into SVs. We found that 1) Kex2p does not cofractionate with Pma1p-containing SVs but peaks in a higher density region of the gradient; 2) shifting sec6 cells from 27 to 38°C has no effect on the fractionation profile of Kex2p; and 3) Drs2p cofractionates with Kex2p in the gradient of 27°C-grown sec6 cells but moves to a lower density region containing the light, Pma1p-positive SVs in 38°C-shifted cells (Supplemental Figure 2). These findings indicate that Drs2p enters the low-density class of SVs. To verify the presence of Drs2p in these vesicles, we next immunoisolated Pma1p-containing membranes from the 38°C gradient peak fractions (6–8 of the Nycodenz gradient in Figure 1). Using protein G-coupled magnetic beads loaded with antibody recognizing the HA-tagged N terminus of Pma1p, we could isolate ∼25% of Pma1p and Drs2p present in the peak fractions (Figure 7B). Preincubation of beads with a peptide corresponding to the HA epitope efficiently blocked the binding of Pma1p- and Drs2p-containing membranes to the beads, demonstrating the specificity of the immunoisolation procedure. From these data we conclude that Drs2p is copackaged with Pma1p in the low-density class of SVs.
Figure 7.
Pma1p-transporting SVs contain Drs2p. (A) Immunoblots of Nycodenz gradient fractions from 38°C-shifted and 27°C-grown sec6-4 cells (same gradients as shown in Figure 1) were probed with antibodies against HA-tagged Pma1p, Drs2p, and the ER membrane protein Dpm1p. (B) Protein G-coupled Dyna beads loaded with anti-HA antibody were used to immuno-isolate Pma1p-HA–containing membranes from plasma membrane-ATPase peak fractions (6–8) of the 38°C-shifted sec6-4 Nycodenz gradient. In one reaction, the beads were preincubated with a peptide corresponding to the HA epitope to control for the specificity of the immunoisolation procedure (+HA peptide). Membranes bound to the beads (B) or collected from the supernatant (S) were analyzed by immunoblotting using antibodies against HA-tagged Pma1p and Drs2p.
Loss of Drs2p and Dnf3p Disrupts PE Asymmetry in Low-Density SVs
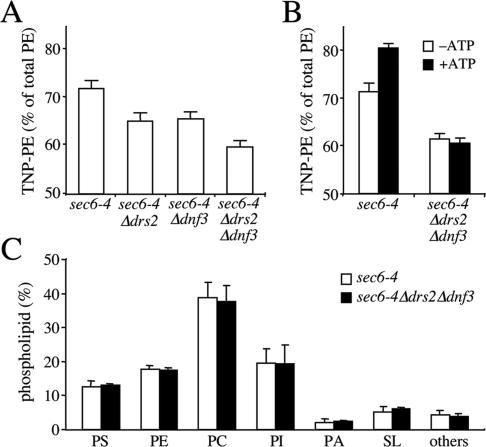
We next investigated whether the Drs2p/Dnf3p-dependent translocase activity defined with NBD phospholipid analogues also regulates the transbilayer distribution of endogenous phospholipids. As an approach, the transbilayer distribution of aminophopholipids in sec6-4 and sec6-4Δdrs2Δdnf3-derived SVs was probed with TNBS, a membrane-impermeable compound reacting with the primary amines in the head group of PE and PS. Unlike PE, membrane-associated PS proved a poor substrate for TNBS labeling and even after a 1-h incubation with sonicated SV-enriched membranes, <10% of PS was converted into TNP-PS (our unpublished data). This led us to focus on the transbilayer distribution of PE. An initial analysis of PE topology was carried out on SV-enriched membranes from 38°C-shifted sec6-4 and sec6-4Δdrs2Δdnf3 cells. The percentage of TNBS-labeled PE in membranes from sec6-4Δdrs2Δdnf3 cells was significantly lower than that in membranes from sec6-4 cells, namely, 60 versus 70% (Figure 8A). Also membranes derived from sec6-4Δdrs2 or sec6-4Δdnf3 cells displayed a significant but smaller reduction in the TNBS-reactive PE pool compared with that in sec6-4 membranes (65 versus 70%). Strikingly, when membranes from sec6-4 cells were preincubated with ATP before TNBS labeling, the percentage of TNBS-labeled PE increased from 70 to 80% (Figure 8B). In contrast, preincubation with ATP had no significant effect on the percentage of TNBS-labeled PE in sec6-4Δdrs2Δdnf3–derived membranes. These strain-dependent differences in PE labeling could not be ascribed to changes in the overall phospholipid composition of the SV-enriched membranes (Figure 8C) and were abolished when membranes were sonicated or reacted with FDNB, a membrane-permeable derivative of TNBS (Supplemental Figure 3). Collectively, these findings suggest that PE is sequestered in the cytosolic leaflet of sec6-4–-derived SVs by a mechanism that requires ATP, Drs2p, and Dnf3p.
Figure 8.
Loss of Drs2p and Dnf3p reduces the TNBS-reactive PE pool in SV-enriched membranes. (A) SV-enriched membranes prepared from 38°C-shifted sec6-4, sec6-4Δdrs2, sec6-4Δdnf3, and sec6-4Δdrs2Δdnf3 cells were incubated with TNBS at 25°C. After 30 min, the reaction was stopped by addition of glycylglycine, and the membranes were subjected to lipid analysis as described in Materials and Methods. Percentages of TNBS-reacted PE relative to total PE are shown as the means ± SD of three independent experiments. (B) SV-enriched membranes from 38°C-shifted sec6-4 and sec6-4Δdrs2Δdnf3 cells were preincubated with or without 2 mM ATP for 30 min at 25°C and then incubated with TNBS in the continuous presence or absence of ATP. After 30 min, the reaction was stopped by addition of glycylglycine, and the membranes were subjected to lipid analysis as described in Materials and Methods. Percentages of TNBS-reacted PE relative to total PE are shown as the means ± SD of two independent experiments. (C) Phospholipid composition of SV-enriched membranes from sec6-4 and sec6-4Δdrs2Δdnf3 cells. Phospholipids were extracted, separated by TLC, and quantified by lipid phosphorus analysis as described in Materials and Methods. Results are expressed as percentage of total phospholipids and represent the means ± SD of two independent experiments. PI, phosphatidylinositol; PA, phosphatidic acid; and SL, sphingolipids.
To obtain more detailed information on the transbilayer PE arrangement in SVs and its dependence on P4 ATPases, SV-enriched membranes from 38°C-shifted sec6-4 and sec6-4Δdrs2Δdnf3 cells were treated with TNBS and then fractionated on Nycodenz gradients. Fractions were analyzed for plasma membrane ATPase activity, phospholipid content, and the percentage of TNBS-reactive PE. Fractionation of membranes from 38°C-shifted sec6-4 and sec6-4Δdrs2Δdnf3 cells in each case resulted in overlapping plasma membrane ATPase and phospholipid peaks that were absent when fractionation was performed on membranes from 27°C-grown cells (Figure 9, top and middle). In fractions containing membranes from 38°C-shifted sec6-4 cells, there was a strong and positive correlation between plasma membrane ATPase levels and the percentage of TNBS-reactive PE, with the latter rising from 50% in ATPase-deficient fractions to nearly 80% in ATPase peak fractions (Figure 9, left bottom). In fractions containing membranes from 38°C-shifted sec6-4Δdrs2Δdnf3 cells, on the other hand, no such correlation was observed, and the percentage of TNBS-reactive PE remained close to 50% in both ATPase-deficient and peak fractions (Figure 9, right bottom). This Drs2p/Dnf3p-dependent change in the TNBS-reactive PE pool was also observed when the analysis was performed on Pma1p-transporting SVs immuno-isolated from ATPase peak fractions (our unpublished data). Together, these results indicate that PE is normally concentrated in the cytosolic leaflet of Pma1p-containing SVs and that maintenance of this PE asymmetry is strictly dependent on Drsp2 and Dnf3p.
Figure 9.
Loss of Drs2p and Dnf3p abolishes PE asymmetry in Pma1p-transporting SVs. Postnuclear supernatants derived from 38°C-shifted or 27°C-grown sec6-4 and sec6-4Δdrs2Δdnf3 cells were incubated with TNBS at 25°C. After 45 min, samples were centrifuged at 13,000 × gav, and glycylglycine was added to the supernatant to stop the reaction. SV-enriched membranes were collected by high-speed centrifugation and then fractionated on a linear Nycodenz/sorbitol gradient as described in the legend to Figure 1. Fractions were collected from the top and analyzed for PM-ATPase activity, lipid phosphorus content, and TNBS-reacted PE. PM-ATPase activity is expressed in arbitrary units based upon the absorbance measured at 660 nm (PM-ATPase) and the TNBS-reacted PE as percentage of the total PE per gradient fraction. Density profiles were similar for both gradients (our unpublished data).
DISCUSSION
Aminophospholipid asymmetry is a universal feature of eukaryotic plasma membranes but precisely how and where this asymmetry is established has remained an open issue. In this study, we have characterized the transbilayer movement and distribution of phospholipids in yeast post-Golgi SVs that mediate cell surface delivery of the plasma membrane protein Pma1p. These vesicles contain an ATP-dependent phospholipid translocase activity that flips NBD-labeled PE, PS, and PC from the luminal to the cytosolic leaflet. This activity is independent of Dnf1p and Dnf2p, two P4 ATPases necessary for inward phospholipid transport across the yeast plasma membrane. Instead, it requires two other P4 ATPases, Drs2p and Dnf3p, that localize primarily to the TGN. Trinitrobenzene sulfonic acid labeling showed that Pma1p-containing SVs have an asymmetric PE arrangement with the bulk of PE (∼80%) located in the cytosolic leaflet. Removal of Drs2p and Dnf3p proved sufficient to abolish this PE asymmetry. Collectively, these results indicate that aminophospholipid asymmetry is created when membrane flows through the Golgi and that P4 ATPases are essential for this process.
Our finding that loss of Dnf1p and Dnf2p has no effect on the SV-associated translocase activity is rather unexpected given that these proteins are required for phospholipid transport across the yeast plasma membrane and must enter SVs to reach their final destination. Previous work revealed that removal of P4 ATPase causes pleiotropic effects on protein trafficking (Chen et al., 1999; Hua et al., 2002; Pomorski et al., 2003). Hence, removal of Dnf1p and Dnf2p may lead to an increased flux of Drs2p and Dnf3p into SVs, essentially replacing Dnf1p and Dnf2p with Drs2p and Dnf3p. Likewise, the strong defect in translocase activity observed in SVs from Δdrs2Δdnf3 mutant cells may reflect a loss of Dnf1p and Dnf2p from the vesicles. Nevertheless, several observations argue in favor of a direct role of Drs2p and Dnf3p in SV-associated translocase activity rather than a role mediated via Dnf1p and Dnf2p. First, removal of Drs2p primarily affects NBD-PS translocation in SVs and has no significant effect on NBD-PC transport, whereas loss of Dnf3p essentially has the opposite effect. In contrast, removal of Dnf1p and Dnf2p in each case affects plasma membrane-associated translocation of NBD-PE, -PS, and -PC to a similar extent (Pomorski et al., 2003). Second, SVs derived from Dnf1,2,3-deficient cells contain a translocase activity recognizing NBD-labeled PS and PE but not NBD-PC. Third, fractionation and immuno-isolation of Pma1p-transporting SVs revealed that they contain Drs2p. Finally, removal of Drs2p and Dnf3p only partially affects NBD-lipid translocation at the plasma membrane (less than 50% reduction for all three lipid analogues; our unpublished data), suggesting that a substantial portion of Dnf1p and Dnf2p enters SVs and reaches the plasma membrane in Δdrs2Δdnf3 mutant cells. Whether the absence of a detectable Dnf1p/Dnf2p-dependent translocase activity in SVs is because of organelle-specific regulation of P4 ATPases and/or reflects differences in their expression remains to be established.
As previously demonstrated for the Dnf1p/Dnf2p-dependent translocase activity at the plasma membrane (Pomorski et al., 2003), our present data indicate that the Drs2p/Dnf3p-dependent translocase activity in SVs is capable of discriminating phospholipids based on their head group and backbone composition. Thus, active transport was limited to a subset of phospholipid analogues that includes NBD-labeled PE, PS, and PC but excludes NBD-labeled SM, phosphatidic acid and phosphatidylglycerol. Strikingly, it seems that Drs2p and Dnf3p are able to distinguish primary aminefrom choline-containing head groups because loss of Drs2p primarily affects NBD-PS transport, whereas loss of Dnf3p primarily affects NBD-PC transport. This is in contrast to Dnf1p and Dnf2p whose removal in each case affects translocation of NBD-labeled PS, PE, and PC to a similar extent (Pomorski et al., 2003). The biological implications of this diversity in head group specificity are unclear. Nevertheless, our findings substantiate the notion that members of the P4 ATPase family do not serve exclusively as aminophospholipid-specific transporters.
Whether P4 ATPases are directly responsible for lipid transport remains to be established. This will require reconstitution of purified ATPases in model membranes. However, compelling evidence for a direct role of P4 ATPases in lipid transport was recently provided with the demonstration that TGN membranes isolated from a yeast Δdnf1,2,3 strain and containing a temperature-sensitive form of Drs2p become defective in NBD-PS translocation when shifted to the nonpermissive temperature (Natarajan et al., 2004). In this study, no ATP-dependent transport activity could be detected when using NBD-PE or NBD-PC with TGN membranes from DRS2 Δdnf1,2,3 or drs2-ts Δdnf1,2,3 strains, suggesting that Drs2p specifically affects PS translocation. Inactivation of Drs2p also blocks formation of a subclass of post-Golgi SVs carrying invertase (Gall et al., 2002), suggesting that the ability of Drs2p to translocate PS to the cytosolic leaflet of the TGN is necessary to support vesicle formation. However, PS-deficient yeast strains deliver proteins normally via the secretory pathway and still require Drs2p to produce invertase-containing SVs (Natarajan et al., 2004). These data suggest that Drs2p translocates another substrate across the TGN membrane with a critical role in vesicle formation. Our present findings indicate that PE is a prime candidate. Thus, we observed that removal of Drs2p reduces NBD-PE translocase activity and causes a partial loss of PE asymmetry in the Pma1p class of SVs. In addition, SVs derived from Dnf1,2,3-deficient cells contain a translocase activity recognizing both NBD-PS and NBD-PE. These data extend our previous finding that cells lacking Drs2p exhibit a partial loss of PE asymmetry at the plasma membrane independently of Dnf1p and Dnf2p (Pomorski et al., 2003). Natarajan et al. (2004) found that the passive movement of NBD-labeled PE to the luminal leaflet of the TGN was considerably slower than that of NBD-PS. This may have affected the ability of these authors to detect Drs2p-dependent translocation of NBD-PE to the cytosolic leaflet of the TGN.
Several lines of evidence point to a functional link between P4 ATPase-dependent lipid transport and the formation of intracellular transport vesicles. Concurrent with a block in lipid transport from the exoplasmic to the cytosolic leaflet of the plasma membrane, cells lacking Dnf1p and Dnf2p exhibit a cold-sensitive defect in the formation of endocytic vesicles (Pomorski et al., 2003), whereas inactivation of Drs2p perturbs both inward lipid transport and the formation of invertase-containing SVs (Gall et al., 2002; Natarajan et al., 2004; this study). Our finding that removal of Drs2p and Dnf3p suffices to eliminate inward lipid transport and PE asymmetry in, but does not block formation of, Pma1p-containing SVs indicates that the link between P4 ATPase-dependent lipid transport and post-Golgi vesicle formation is not absolute. Drs2p is essential for cell growth at 23°C or below (Ripmaster et al., 1993; Siegmund et al., 1998). Hence, analogous to the role of Dnf1p and Dnf2p in endocytic vesicle formation at the plasma membrane, it is feasible that Drs2p/Dnf3p-dependent lipid transport is critical for budding Pma1p-transporting SVs only at low temperature when a decreased fluidity of the membrane may prevent coat assembly from driving this process alone.
Precisely how P4 ATPase-dependent lipid transport participates in vesicle biogenesis remains to be established. It seems that not so much an asymmetric distribution of particular lipid species per se but rather the physical displacement of lipid from the exoplasmic to the cytosolic leaflet is important. As discussed above, PS is not an obligatory substrate for the role Drs2p plays in protein transport (Natarajan et al., 2004). This rules out the possibility that an asymmetric distribution of PS and the recruitment of PS-binding proteins to the TGN are required for SV formation. Moreover, we previously showed that removal of multiple P4 ATPases causes a marked decrease in the aminophospholipid content of cellular membranes (Pomorski et al., 2003). This finding is hard to reconcile with the idea that maintenance of a high concentration of aminophospholipids in the cytosolic leaflet accounts for the requirement of P4 ATPases in vesicle formation, because down-regulation of aminophospholipid levels would have a counterproductive effect. Instead, P4 ATPases may play a more direct and mechanistic role in vesicle formation. Budding of a transport vesicle requires membrane bending, which in turn must be associated with an imbalance in surface area between the two leaflets. Thus, in the absence of vesicle fusion, vesicle budding would increase the ratio in surface area between the cytosolic and exoplasmic leaflet in the donor organelle. Within the framework of the bilayer-couple hypothesis (Sheetz and Singer, 1974), this would induce a negative curvature that progressively inhibits the formation of new vesicles. P4 ATPase-dependent lipid transport might serve to relax this negative curvature and hence support ongoing vesiculation (Graham, 2004). Alternatively, rather than simply relaxing negative curvature, P4 ATPases might use the energy of ATP hydrolysis to drive vesicle budding by creating a positive curvature (Devaux, 2000). In support of the latter model, stimulation of the aminophospholipid translocase activity in red blood cells provokes the formation of endocytic vesicles (Birchmeier et al., 1979; Muller et al., 1994), even though this cell type lacks the machinery for generating coated vesicles. Regardless, a mechanistic role for P4 ATPases in vesicle budding would better fit the variation in head group specificity observed among the different family members. Aminophospholipid asymmetry would then merely be a consequence of the fact that without any lipid selectively, P4 ATPases would be trapped in a futile cycle of ATP-consuming lipid transport.
Supplementary Material
Acknowledgments
We are grateful to Todd Graham for generously providing the anti-Drs2p antibody and to Gerrit van Meer and Andreas Herrmann for stimulating discussions. This work was supported by grants from the Schering Foundation (to N.A.-B.), the Deutsche Forschungsgemeinschaft (to T. P.), the Meelmeijer foundation (to Q. L.), and the Dutch Organization of Sciences (NWO-CW) (to J. H.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-10-0912) on February 1, 2006.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bell, R. M., Ballas, L. M., and Coleman, R. A. (1981). Lipid topogenesis. J. Lipid Res. 22, 391–403. [PubMed] [Google Scholar]
- Birchmeier, W., Lanz, J. H., Winterhalter, K. H., and Conrad, M. J. (1979). ATP-induced endocytosis in human erythrocyte ghosts. Characterization of the process and isolation of the endocytosed vesicles. J. Biol. Chem. 254, 9298–9304. [PubMed] [Google Scholar]
- Bishop, W. R., and Bell, R. M. (1985). Assembly of the endoplasmic reticulum phospholipid bilayer: the phosphatidylcholine transporter. Cell 42, 51–60. [DOI] [PubMed] [Google Scholar]
- Chen, C. Y., Ingram, M. F., Rosal, P. H., and Graham, T. R. (1999). Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 147, 1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribier, S., Morrot, G., and Zachowski, A. (1993). Dynamics of the membrane lipid phase. Prostaglandins Leukot. Essent. Fatty Acids 48, 27–32. [DOI] [PubMed] [Google Scholar]
- Cunningham, K. W., and Wickner, W. T. (1989). Yeast KEX2 protease and mannosyltransferase I are localized to distinct compartments of the secretory pathway. Yeast 5, 25–33. [DOI] [PubMed] [Google Scholar]
- Devaux, P. F. (2000). Is lipid translocation involved during endo- and exocytosis? Biochimie 82, 497–509. [DOI] [PubMed] [Google Scholar]
- Gall, W. E., Geething, N. C., Hua, Z., Ingram, M. F., Liu, K., Chen, S. I., and Graham, T. R. (2002). Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 12, 1623–1627. [DOI] [PubMed] [Google Scholar]
- Gomes, E., Jakobsen, M. K., Axelsen, K. B., Geisler, M., and Palmgren, M. G. (2000). Chilling tolerance in Arabidopsis involves ALA1, a member of a new family of putative aminophospholipid translocases. Plant Cell 12, 2441–2454. [PMC free article] [PubMed] [Google Scholar]
- Graham, T. R. (2004). Flippases and vesicle-mediated protein transport. Trends Cell Biol. 14, 670–677. [DOI] [PubMed] [Google Scholar]
- Harsay, E., and Schekman, R. (2002). A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J. Cell Biol. 156, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, Z., Fatheddin, P., and Graham, T. R. (2002). An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell 13, 3162–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg, R. D., and McConnell, H. M. (1971). Inside-outside transitions of phospholipids in vesicle membranes. Biochem. 10, 1111–1120. [DOI] [PubMed] [Google Scholar]
- Kubelt, J., Menon, A. K., Muller, P., and Herrmann, A. (2002). Transbilayer movement of fluorescent phospholipid analogues in the cytoplasmic membrane of Escherichia coli. Biochem. 41, 5605–5612. [DOI] [PubMed] [Google Scholar]
- Levine, T. P., Wiggins, C. A., and Munro, S. (2000). Inositol phosphorylceramide synthase is located in the Golgi apparatus of Saccharomyces cerevisiae. Mol. Biol. Cell 11, 2267–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx, U., Polakowski, T., Pomorski, T., Lang, C., Nelson, H., Nelson, N., and Herrmann, A. (1999). Rapid transbilayer movement of fluorescent phospholipid analogues in the plasma membrane of endocytosis-deficient yeast cells does not require the Drs2 protein. Eur. J. Biochem. 263, 254–263. [DOI] [PubMed] [Google Scholar]
- Muller, P., Pomorski, T., and Herrmann, A. (1994). Incorporation of phospholipid analogues into the plasma membrane affects ATP-induced vesiculation of human erythrocyte ghosts. Biochem. Biophys. Res. Commun. 199, 881–887. [DOI] [PubMed] [Google Scholar]
- Natarajan, P., Wang, J., Hua, Z., and Graham, T. R. (2004). Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc. Natl. Acad. Sci. USA 101, 10614–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomorski, T., Holthuis, J. C., Herrmann, A., and van Meer, G. (2004). Tracking down lipid flippases and their biological functions. J. Cell Sci. 117, 805–813. [DOI] [PubMed] [Google Scholar]
- Pomorski, T., Lombardi, R., Riezman, H., Devaux, P. F., van Meer, G., and Holthuis, J. C. (2003). Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14, 1240–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripmaster, T. L., Vaughn, G. P., and Woolford, J. L., Jr. (1993). DRS1 to DRS7, novel genes required for ribosome assembly and function in Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 7901–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, K., Fujimura-Kamada, K., Furuta, N., Kato, U., Umeda, M., and Tanaka, K. (2004). Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 3418–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret, M., and Devaux, P. F. (1984). ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. USA 81, 3751–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz, M. P., and Singer, S. J. (1974). Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. USA 71, 4457–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund, A., Grant, A., Angeletti, C., Malone, L., Nichols, J. W., and Rudolph, H. K. (1998). Loss of Drs2p does not abolish transfer of fluorescence-labeled phospholipids across the plasma membrane of Saccharomyces cerevisiae. J. Biol. Chem. 273, 34399–34405. [DOI] [PubMed] [Google Scholar]
- Tang, X., Halleck, M. S., Schlegel, R. A., and Williamson, P. (1996). A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 272, 1495–1497. [DOI] [PubMed] [Google Scholar]
- Wicky, S., Schwarz, H., and Singer-Kruger, B. (2004). Molecular interactions of yeast Neo1p, an essential member of the Drs2 family of aminophospholipid translocases, and its role in membrane trafficking within the endomembrane system. Mol. Cell. Biol. 24, 7402–7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachowski, A., Henry, J. P., and Devaux, P. F. (1989). Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature 340, 75–76. [DOI] [PubMed] [Google Scholar]
- Ziman, M., Chuang, J. S., and Schekman, R. W. (1996). Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol. Biol. Cell 7, 1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.