Abstract
Tat, the transactivator of HIV-1 gene expression, is released by acutely HIV-1-infected T-cells and promotes adhesion, migration, and growth of inflammatory cytokine-activated endothelial and Kaposi's sarcoma cells. It has been previously demonstrated that these effects of Tat are due to its ability to bind through its arginine-glycine-aspartic (RGD) region to the α5β1 and αvβ3 integrins. However, the signaling pathways linking Tat to the regulation of cellular functions are incompletely understood. Here, we report that Tat ligation on human endothelial cells results in the activation of the small GTPases Ras and Rac and the mitogen-activated protein kinase ERK, specifically through its RGD region. In addition, we demonstrated that Tat activation of Ras, but not of Rac, induces ERK phosphorylation. We also found that the receptor proximal events accompanying Tat-induced Ras activation are mediated by tyrosine phosphorylation of Shc and recruitment of Grb2. Moreover, Tat enabled endothelial cells to progress through the G1 phase in response to bFGF, and the process is linked to ERK activation. Taken together, these data provide novel evidence about the ability of Tat to activate the Ras-ERK cascade which may be relevant for endothelial cell proliferation and for Kaposi's sarcoma progression.
INTRODUCTION
Tat, the transactivator of human immunodeficiency virus type-1 (HIV-1) gene expression, is released by acutely HIV-1-infected T-cells and, in this extracellular form, promotes the adhesion, migration, invasion, and growth of Kaposi's sarcoma (KS) and endothelial cells activated with T helper-1 type inflammatory cytokines (such as interferon-γ, interleukin 1-β, and tumor necrosis factor-α; Ensoli et al., 1990; Barillari et al., 1992, 1993, 1999a; Ensoli et al., 1993; Fiorelli et al., 1995, 1998, 1999; Samaniego et al., 1995, 1997, 1998). In HIV-1-infected individuals, KS is more frequent and aggressive, with several lines of evidence indicating that this is due to the Tat protein acting synergistically with basic fibroblast growth factor (bFGF) to induce angiogenesis, vascular permeability, and edema. These latter activities are the main processes involved in KS progression (Ensoli et al., 1994; Barillari et al., 1999b; Toschi et al., 2001). Five distinct functional domains have been characterized in the Tat protein: N-terminal, cystein-rich, core, basic, and C-terminal. We and others have previously demonstrated that the multiple paracrine effects of Tat are principally due to its C-terminal domain containing an arginine-glycine-aspartic (RGD) sequence, which represents the principal cell attachment moiety recognized by integrin receptors. In fact, this Tat domain can bind with high affinity to integrins α5β1 and αvβ3, which are receptors for fibronectin (FN) and vitronectin, respectively, that are highly expressed in KS associated with the acquired immune-deficiency syndrome (AIDS-KS; Barillari et al., 1993, 1994; Ensoli et al., 1994).
Integrins are among the major receptors connecting cells to the surrounding extracellular matrix. This extracellular matrix is three-dimensional, complex, and dynamic in its molecular composition (Even-Ram and Yamada, 2005). Engagement of integrins during cell adhesion regulates migration, tissue organization, matrix remodelling, and, in concert with receptors for soluble factors, survival, differentiation, and proliferation. In addition, integrin-mediated cell adhesion elicits signaling pathways that may be involved in angiogenesis (Eliceiri and Cheresh, 2001). Integrin α5β1-mediated cell adhesion to FN is specifically efficient in supporting mitogen-dependent proliferation of endothelial cells (Danen and Yamada, 2001). In fact, FN can decrease the growth factor requirement for DNA synthesis up to 1000-fold (Asthagiri et al., 1999). However, the ligation of both αvβ3 and growth factor receptors on vascular cells is essential for the sustained activation of mitogen-activated protein kinases (MAPK) during angiogenesis (Eliceiri et al., 1998). In many nonimmortalized cells, it has been shown that engagement of either αvβ3, β4, or several β1 integrins determines Shc phosphorylation, which combines with the Grb2/SOS complex, causing activation of the Ras-ERK/MAPK cascade (Mainiero et al., 1995, 1997, 1998; Wary et al., 1996). Furthermore, the simultaneous stimulation of Ras by Shc-linked integrins and growth factor receptors seems to be key event in order to activate ERK to the extent required for cell cycle progression (Lai and Pawson, 2000).
The addition of extracellular Tat in culture elicits different signal transduction pathways, including focal adhesion kinase (FAK)-associated phosphoinositide (PI)-3 kinase, and the activation of MAPKs, such as ERK and c-Jun N-terminal kinase (Milani et al., 1996, 1998; Ganju et al., 1998; Kumar et al., 1998; Mischiati et al., 1999; Rusnati et al., 2001). Tat is also able to bind and phosphorylate the vascular endothelial growth factor receptor type 2 likely through its basic domain, which contains a sequence similar to those of the vascular endothelial growth factor-A and other growth factors, such as fibroblast growth factor, hepatocyte growth factor, and heparin-binding epidermal growth factor (Albini et al., 1996). In contrast, the basic region of Tat binds only to the low-affinity binding sites of vascular endothelial growth factor receptor type 2 expressed by endothelial cells, thus showing a reduced ability to activate endothelial cells in vitro and to induce angiogenic activity in vivo (Mitola et al., 2000). Furthermore, full activation of the angiogenic program requires the Tat C-terminal region containing the RGD sequence (Mitola et al., 2000).
Because Tat mimics extracellular matrix proteins, such as FN and vitronectin, and binds α5β1 and αvβ3 integrins through its RGD domain, we investigated whether Tat induces the Ras/MAPK pathway in primary human umbilical vascular endothelial cells (HUVEC) and immortalized endothelial cells (EA-hy 926). Our studies revealed that Tat induces the activation of Ras and the phosphorylation of ERK1/2 through its RGD sequence. Further experiments demonstrated that these effects are mediated by the phosphorylation of Shc via the recruitment of its adaptor, Grb2. Finally, attachment and spreading on Tat, as well as on FN, in the presence of a mitogen, such as bFGF, which is known to actively synergize with Tat (Ensoli et al., 1994; Barillari et al., 1999a), induced the expression of Cyclin D1 and then progression of endothelial cells through G1.
MATERIALS AND METHODS
Antibodies and Other Reagents
Recombinant HIV-1 Tat protein (from the III B isolate) was expressed, purified, and handled as previously described (Ensoli et al., 1990, 1993, 1994; Chang et al., 1997). The [46-60] and [66-80]Tat peptides mapping in the basic and RGD regions, respectively, and the [1-20]Tat and [57-70]Tat control peptides were synthesized by UFPEPTIDES (Ferrara, Italy). Recombinant human FN was purchased from Roche (Indianapolis, IN).
The monoclonal antibody (mAb) to phospho-ERK was purchased from Cell Signaling Technology (Beverly, MA). Rabbit antibodies to the SH2 domain of Shc and the anti-Grb2 mAb were from BD Transduction Laboratories (Lexington, KY). The rabbit anti-ERK and the mouse anti-Shc antibodies, used as controls for equal loading, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-Tyr(P) mAb 4G10 and the anti-Ras antibody were purchased from Upstate Biotechnology (Lake Placid, NY). Secondary anti-mouse and anti-rabbit horseradish peroxidase antibodies were obtained from Amersham Pharmacia Biotech (Little Chalfont, United Kingdom). The 5′-bromo-2′-deoxy-uridine (BrdU) was from Sigma (St. Louis, MO), the mouse anti-BrdU was obtained from Chemicon (Temecula, CA), and AP-conjugated anti-mouse IgGs from Dako (Glostrup, Denmark). The blocking mAbs against α5β1 (clone JBS5), αvβ3 (clone LM609) integrins were purchased from Chemicon (Temecula, CA); the blocking mAb anti-α4β1 (clone P4G9) integrin was from Società Italiana Chimici (Rome, Italy). The mAb major histocompatibility complex (MHC) class I (clone W6.32) was obtained from Schering Plough (Dardilly, France). The p38 MAPK inhibitor SB 203580 was purchased from Alexis (San Diego, CA) and the MEK-1 inhibitor PD 98059 was from Calbiochem (Darmstadt, Germany). The mAb anti-Cyclin D1 (clone DCS-6) was obtained from Neomarkers (Westinghouse, CA). The expression vector for the dominant negative kinase-dead version of MEK (MEK-K97M) was kindly provided by Dr. Bernard Baumann. The expression vector for the dominant negative H-Ras (S17N mutant) and the siRNA oligonucleotides for Rac or Ras were purchased from Upstate Biotechnology, which also provided the Scramble Duplex negative control.
Cell Culture and Stimulation
HUVECs were cultured as described (Barillari et al., 1999a; Fiorelli et al., 1999) and used between passages 3 and 6. The EA-hy 926 cell line, an immortalized cell hybrid obtained upon cell fusion of HUVEC and the human lung adenocarcinoma cell line A549, retains most markers of human endothelial cells (Edgell et al., 1983). EA-hy 926 cells were cultured in DMEM (Invitrogen, Gaithersburg, MD), supplemented with 10% fetal bovine serum (FBS; Euroclone, United Kingdom), 100 μg/ml streptomycin, 100 U/ml penicillin (Invitrogen, Gaithersburg, MD), and l-glutamine (Invitrogen) at 37°C in a 5% CO2 incubator. Starvation involved incubation of the HUVEC and EA-hy 926 cells in Medium 199 (Invitrogen) without fetal calf serum for 24 and 36 h, respectively. After starvation, equal amounts of cells were incubated for different time periods with polystyrene beads (2.5-μm diameter; Interfacial Dynamics, Portland, OR) coated with either the indicated amounts of recombinant Tat, or equimolar amounts of Tat peptides, or of human plasma FN, or BSA, as described (Mainiero et al., 1995, 1997, 1998; Wary et al., 1996).
In some experiments, the cells were preincubated with blocking mAbs against the α5β1, αvβ3, and α4β1 integrins, and the anti-MHC class I (W6.32) served as controls. After a 30-min incubation at 4°C, the cells were then stimulated for 10 min with polystyrene beads coated with the indicated doses of recombinant Tat, as previously described.
Biochemical Methods
Ras activity was assessed by employing the Ras activation assay kit (Upstate Biotechnology). Briefly, treated cells were lysed in a buffer containing 50 nM TrisHCl, pH 7.5, NaCl 150 nM, 1% Triton X-100, 0.15% Na deoxycholate, Na orthovanadate 1 mM, Na pyrophosphate 50 nM, NaF 100 mM, EDTA 1 mM, EGTA 1 mM, MgCl2 10 mM, and a protease inhibitor mixture. The cell lysates (600 μg) were incubated with 10 μl of glutathione S-transferase (GST)-Raf-Ras binding domain (RBD) coupled to agarose for 30 min at 4°C. The beads were washed with the lysis buffer and resuspended in 2× SDS sample buffer. Western blotting was performed with the anti-Ras antibody (Upstate Biotechnology). Rac activity was similarly determined by using the Rac activation assay kit (Upstate Biotechnology). The optical density of the bands (integrated area, arbitrary units, AU) was measured by GS-800 Imaging Densitometer (Bio-Rad Laboratories, Hercules, CA).
To examine ERK activity, cells were extracted with Triton lysis buffer (1% Triton X-100, NaCl 150 nM, CaCl2 1 mM, MgCl2 1 mM, NaF 10 mM, iodoacetamide 10 mM) containing phosphatase and protease inhibitors for 30 min on ice. Equal amounts of total proteins were boiled in sample buffer and separated by SDS-PAGE. After immunoblotting with an ERK phospho-specific antibody, immunoreactive bands were visualized by using horseradish peroxidase-conjugated secondary antibody and the ECL system (Amersham Pharmacia Biotech).
To immunoprecipitate Shc, stimulated and unstimulated endothelial cells were extracted in Triton lysis buffer as described above for 30 min on ice. Equal amounts of total protein were then incubated with mouse anti-Shc antibody overnight at 4°C. The antibody-antigen complexes were immunoprecipitated by incubation for 1 h at 4°C with 30 μl of protein A-agarose (Pierce, Rockford, IL). Bound proteins were solubilized in 2× SDS PAGE sample buffer and further analyzed by immunoblotting.
Transfection of EA-hy 926 Cells
The EA-hy 926 cells, cultured in DMEM supplemented with 10% FBS, were transiently transfected with 1 μg of H-Ras cDNA or MEK-97M expressing vector using Lipofectin reagent (Invitrogen, Life Technology, Gaithersburg, MD), in accordance with the manufacturer's instructions. In parallel experiments the EA-hy 926 cells were transfected with 200 nM siRNA duplex in DMEM serum-free using Oligofectamine reagent (Invitrogen), in accordance with the manufacturer's instructions. Cell lysates were analyzed for levels of H-Ras or Rac protein by Western blotting with the specific antibodies previously described.
Measurement of Cell Cycle Progression
To monitor progression through G1 and entry in S phase, cells treated or not with Tat or FN were analyzed through 5′-bromo-2′-deoxy-uridine (BrdU) incorporation and Cyclin D1 expression by western blot. HUVEC and EA-hy 926 cells plated at low density on chamber slides coated with 25 μg/ml type I collagen were synchronized in G0 by growth factor starvation for either 24 or 36 h, respectively. The cells were then incubated for 24 h with 10 μg/ml Tat, 25 μg/ml FN, or 5 μg/ml BSA in defined medium (M199 supplemented with 25 ng/ml bFGF, 1 μg/ml heparin, 6.25 μg/ml insulin, 1.25 mg/ml BSA) containing 10 μM BrdU. For Cyclin D1 expression, HUVEC and EA-hy 926 cells were synchronized and stimulated as described above. The cells were then lysed in Triton lysis buffer containing 0.1% SDS for 30 min on ice and then analyzed by Western blotting.
To investigate whether Tat induces entry in S phase specifically through ERK activation, the cells were serum-starved and pretreated with the above-described medium containing either 25 μM PD 98059 (Calbiochem) or 10 μM SB 203580 (Alexis), before stimulation for 24 h with Tat and dimethyl sulfoxide (DMSO) vehicle. In parallel experiments, the cells were transiently transfected with 1 μg of MEK-97M expressing vector and then stimulated with Tat or FN, as positive control. After fixation with cold methanol, cells were stained with anti-BrdU mAb and AP-conjugated anti-mouse IgGs (Dako). Cells were visualized using an Axioskop 2 plus microscope (Zeiss, Jena, Germany) using AxioVision 3.06 software (Zeiss) under 40× original magnification (see Figures 6 and 7 and Supplementary Figure 2). Images were captured and processed using an Axiocam camera (Zeiss). BrdU-labeled nuclei were assessed and quantified as percent total by using a Zeiss image analysis system (software KS 300, version 3.2).
Figure 6.
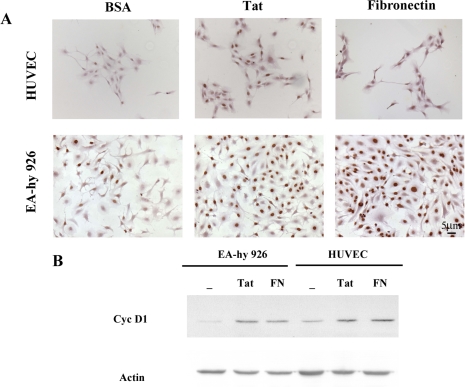
Tat promotes endothelial cell cycle progression through the induction of Cyclin D1 expression. (A) HUVEC and EA-hy 926 cells were plated on type I collagen, synchronized in G0, and incubated for 24 h in a medium containing 25 ng/ml bFGF in the presence of 10 μg/ml Tat, or 25 μg/ml FN or BSA as control. Entry into the S phase was examined by 5′-bromo-2′-deoxy-uridine (BrdU) incorporation and anti-BrdU staining. A consistent amount of HUVECs incubated with Tat (18%) or FN (19%), compared with BSA (2%), entered into the S phase during the 24 h of the assay. Similarly, stimulation of EA-hy 926 cells with Tat and FN induced 40 and 67% of the cells to incorporate BrdU, whereas only 13% of the cells incubated in the presence of BSA entered the S phase. (B) HUVEC and EA-hy 926 cells synchronized in G0 were treated as described above. Immunoblotting with anti-cyclin D1 antibody was then performed on equal amounts of total proteins obtained from the cell lysates.
Figure 7.
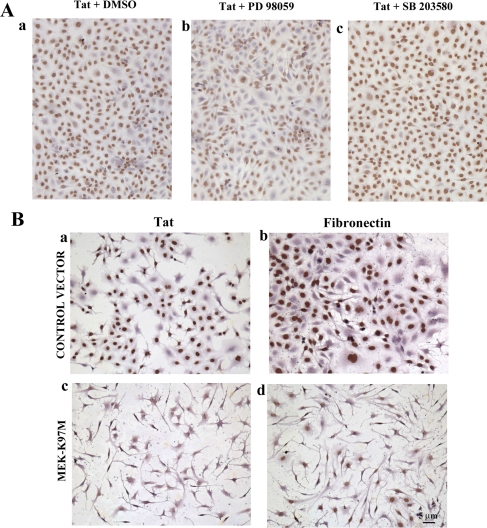
Tat promotes endothelial cell cycle progression through ERK1/2 activation. The EA-hy 926 cells were seeded on type I collagen and preincubated with 25 μM PD 98059 or 10 μM SB 203580 for 20 min. Cells were then stimulated with 10 μg/ml Tat for 24 h and pulsed with BrdU. (A) A reduced percentage of cells incubated with PD 98059 and Tat (61%, b) entered into the S phase compared with the percentage of cells incubated with Tat and DMSO (80%, a) or SB 203580 and Tat (82%, c). The role of ERK1/2 was further analyzed in EA-hy 926 cells transfected with the kinase-dead MEK-K97M and then incubated for 24 h in a medium containing bFGF, and Tat or FN. (B) Entry into the S phase was monitored by BrdU incorporation, as described above. Only 8% of the cells transfected and treated with Tat incorporated BrdU compared with 70% of the cells transfected with the control vector (a and c). Similar results have been obtained in cells treated with FN (b and d).
RESULTS
Activation of Ras and Rac upon Stimulation of Endothelial Cells with HIV-1 Tat
The small GTPases Ras and Rac control signaling pathways that are key regulators of several aspects of normal cell growth and malignant transformation. In particular, they can be activated by many cell-surface receptors, including integrins, and are important elements in cellular signaling pathway, such as the activation of the MAPK/ERK signaling cascade (Kinbara et al., 2003).
Ras-GTP pulldown experiments were therefore performed to examine if stimulation of endothelial cells with Tat resulted in activation of Ras. In particular, active GTP-bound Ras was pulled down from lysates of EA-hy 926 cells with the GST-Raf-RBD coupled to glutathione agarose, and activated Ras was determined by immunoblotting with a Ras antibody. The cells, starved for 36 h in serum-free medium were stimulated with 1 μg of Tat conjugated to polystyrene beads. As a positive control, the cells were stimulated with FN, whereas BSA was used as negative control. Both positive and negative controls were conjugated to polystyrene beads following the same procedure used for Tat. We found that Tat rapidly activates Ras within 1 min; the activation persists at 3 min and starts to decrease at 5 min (Figure 1). The results of this experiment show that stimulation of endothelial cells with Tat causes activation of Ras, thus, suggesting its possible involvement in the signaling pathways induced by Tat.
Figure 1.
Activation of Ras and Rac upon stimulation of endothelial cells with Tat. Active GTP-bound Ras was pulled down from lysates of EA-hy 926 cells with GST-Raf-RBD coupled to glutathione agarose, and Ras was determined by immunoblotting with a Ras antibody. Tat rapidly activates Ras within 1 min; the activation is still persistent at 3 min and starts to decrease at 5 min. A pulldown assay with a GST-fusion protein corresponding to the p21-binding domain of PAK-1 was performed on EA-hy 926 cells stimulated for different times with Tat. Activation of Rac by Tat, FN, or BSA is shown in the top panel. The bottom panels of each set of immunoblots represent either total Ras or total Rac present in the cells lysates.
Previous studies provided evidence that the ligation of integrin α5β1 caused significant activation of Rac in endothelial cells (Mettouchi et al., 2001). To examine the ability of Tat to induce activation of Rac, a pulldown assay with a GST-fusion protein corresponding to the binding domain of the p21-activated kinase-1 was performed on EA-hy 926 cells stimulated with 1 μg of Tat conjugated to polystyrene beads for different times. The results show that Rac is rapidly activated by Tat within 1 min and the signal starts to decrease between 3 and 5 min (Figure 1). By contrast, the activation of Rac induced by FN is still elevated at 5 min, thus suggesting that the more persistent stimulation of Rac determined by FN might be due not only to its binding to α5β1 but also to other integrin receptors, such as α4β1 (Figure 1). Taken together, these results demonstrate that Tat induces the activation of both Ras and Rac in human endothelial cells.
Tat Induces ERK1/2 Phosphorylation, through Its RGD Region, in Endothelial Cells
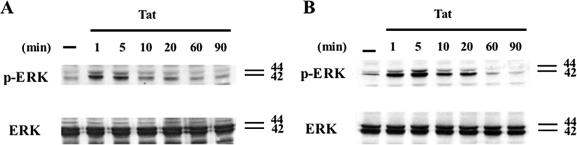
ERK is an important member of the MAPK family that acts as downstream mediator of small GTPases signaling cascades induced by integrin-mediated cell adhesion to the extracellular matrix and growth factor receptors (Giancotti and Ruoslahti, 1999). Because it has been previously observed that Tat is able to activate MAPKs (Ganju et al., 1998; Mischiati et al., 1999; Rusnati et al., 2001), we first examined whether the phosphorylation of ERK was induced also in our cellular models upon treatment with Tat. Further, both HUVEC and EA-hy 926 cells, starved for 24 or 36 h in serum-free medium, respectively, were stimulated with 1 μg of Tat conjugated to polystyrene beads for different times. ERK activation was then analyzed by Western blot analysis using an antibody against the active phosphorylated form of this MAPK. As shown in Figure 2A, 1 μg of Tat was able to increase ERK1/2 phosphorylation in HUVEC cells at 1 min, reached a peak at 5 min, and then started to decrease. To confirm that the optimal dose of Tat able to induce ERK phosphorylation is 1 μg, we also performed a titration experiment by using 0.5, 1, and 5 μg of recombinant protein (Supplementary Figure 1). ERK activation was induced by 1 μg of Tat also in EA-hy 926 cells; however, the phosphorylation was more persistent than that observed in HUVECs (Figure 2B). These data indicate that ligation of Tat on human endothelial cells causes a rapid and significant activation of ERK1/2.
Figure 2.
Time-course analysis of ERK1/2 activation induced by Tat in HUVEC and EA-hy 926 cells. HUVEC (A) and EA-hy 926 (B) cells starved for 24 and 36 h in serum-free medium, respectively, were stimulated with Tat conjugated to polystyrene beads for different time points. The bottom panels of each set of immunoblots represent total ERK present in the cell lysates.
Previous studies by us and others have indicated the important role of the basic and RGD domains of Tat in promoting vascular cell locomotion and growth in vitro and angiogenesis in vivo (Barillari et al., 1999a; Mitola et al., 2000). To identify the domain/s of Tat responsible of the activation of ERK1/2 in endothelial cells, experiments were performed using the [46-60] and [66-80]Tat peptides mapping in the basic and RGD region, respectively. [1-20]Tat and [57-70]Tat peptides, which are not involved in the angiogenic effect of the protein, were used as controls. FN and BSA were used as positive and negative controls, respectively. Immunoblotting with anti-phosphoERK antibody indicated that the activation of ERK1/2 in HUVECs is mainly mediated by the [66-80]Tat domain which contains the RGD region (Figure 3A). By contrast, [46-60]Tat peptide containing the basic sequence as well as the other two control peptides do not induce any significant phosphorylation of ERK (Figure 3A). To further study the role of the RGD region of Tat in inducing ERK phosphorylation, additional experiments have been performed by using different amounts (1 or 2 μg) of blocking antibodies against integrins αvβ3, α5β1, and α4β1 and the anti-MHC class I (anti-W6.32) as control IgG1 antibody. The results indicated that ERK1/2 activation by Tat is efficaciously reduced only by the treatment with 2 μg of the antibodies against α5β1 and αvβ3. However, cell preincubation with 1 μg of both antibodies combined acts synergistically in blocking ERK1/2 phosphorylation by Tat (Figure 3B). Moreover, blocking of the integrin α4β1, which binds the CS1 region of FN, does not significantly influence ERK activation promoted by Tat (Figure 3B). All together, these results strongly demonstrate the specific role of the RGD sequence of Tat in sustaining ERK1/2 phosphorylation.
Figure 3.
Tat promotes, through its RGD region, ERK1/2 activation on endothelial cells. (A) HUVECs were stimulated with the [46-60] and [66-80]Tat peptides mapping in the basic and RGD region, respectively. [1-20]Tat and [57-70]Tat peptides were used as controls. As a positive control, the cells were stimulated with FN, whereas BSA was used as negative control. Immunoblotting with anti-phosphoERK antibody was then performed on equal amounts of total proteins obtained from the cell lysates, as shown in the bottom panel. (B) The cells were pretreated with two different doses (1 or 2 μg/106 cells) of the blocking mAbs against integrins α5β1, αvβ3, α4β1, or a mAb anti-MHC class I as control and were then stimulated with 1 μg of Tat. Immunoblotting with anti-phosphoERK antibody on cell lysates indicates that ERK1/2 activation by Tat is decreased by treatment with 2 μg of the antibodies against α5β1 and αvβ3. Combination of the antibodies against α5β1 and αvβ3 (1 μg each) synergistically reduces ERK phosphorylation induced by Tat. In contrast, both doses of mAb anti-α4β1 do not significantly influence the activation of ERK promoted by Tat. Intensities of both 42 and 44 phosphorylated bands of ERK1/2 (arbitrary units, AU) were measured and values are given on bottom of the top panels. An equal amount of ERK was used in this analysis (bottom panel).
Tat Promotes ERK Phosphorylation in Endothelial Cells via the Activation of Ras
To better define the role of Ras and Rac and their eventual link to ERK in the signaling cascade induced by Tat, we transiently transfected EA-hy 926 cells with a dominant-negative Ras (S17N). On transfection, the cells were starved and then stimulated with the previously established amounts of recombinant Tat conjugated with polystyrene beads for 5 min. Finally, activation of ERK was analyzed by immunoblotting as previously described. As shown in Figure 4A the introduction of this dominant-negative prevented the phosphorylation of ERK1/2 in response to the treatment with Tat, thus suggesting a crucial role of Ras in the MAP kinase pathway induced by Tat. To further strengthen these data and clarify the role of Rac, we used an interference approach (Dykxhoorn et al., 2003). To suppress the expression of the endogenous Ras and Rac genes, EA-hy 926 cells were transiently transfected with siRNAs previously identified for their efficiency to target key sites of the mRNA encoding for Ras and Rac, respectively (Upstate/Dharmacon). The cells were then treated with Tat, and ERK phosphorylation was analyzed as previously described. In accordance with the data obtained through the transfection with the dominant-negative, when Ras is silenced ERK phosphorylation is decreased and no induction is observed after stimulation with Tat (Figure 4B). By contrast, the silencing of Rac causes a reduction of its expression, but this has a smaller effect on the phosphorylation of ERK1/2 induced by Tat, with levels similar to those obtained in control cells (Figure 4C). Taken together, these results demonstrate that, in our cellular model, Tat activation of Ras, and not of Rac, is linked to the phosphorylation of ERK.
Figure 4.
ERK phosphorylation by Tat is induced via activation of Ras. (A) EA-hy 926 cells were transiently transfected with a dominant-negative of Ras (S17N) that prevented the phosporylation of ERK1/2 induced by Tat. To confirm these data and to clarify the role of Rac, EA-hy 926 cells were transiently transfected with siRNAs previously identified for their efficiency to target key sites of the mRNA encoding for Ras or Rac. (B) The silencing of Ras decreased ERK1/2 phosphorylation and no induction was observed after stimulation with Tat. (C) In contrast, the silencing of Rac caused a reduction of its expression, but had only a small effect on phosphorylation of ERK1/2 stimulated by Tat. The blots were stripped and reprobed with anti-ERK antibody to confirm equal loading (middle panels). Intensities of both 42 and 44 phosphorylated bands of ERK1/2 (arbitrary units, AU) were measured and values are given on bottom of the top panels.
Tat Induces Shc Phosphorylation and Recruitment of its Adaptor, Grb2
Biochemical and genetic evidence support the role of Shc in integrin-mediated activation of the Ras/MAPK pathway. In fact, it has been previously observed that integrins that do not activate Shc are weak activators of ERK in primary fibroblasts, endothelial cells, and keratinocytes (Wary et al., 1996; Mainiero et al., 1997; Pozzi et al., 1998). To further characterize the upstream cascade leading to Ras then ERK activation, we determined if Tat regulates the tyrosine phosphorylation of Shc and recruitment of its adaptor protein, Grb2. Equal amounts of total proteins from either HUVEC or EA-hy 926 cells treated with Tat were immunoprecipitated with anti-Shc followed by immunoblotting with either anti-phosphotyrosine or anti-Grb2 antibodies. As shown in Figure 5, A and B, Tat induces Shc phosphorylation on tyrosine residues and the association of Shc with Grb2 within 5 min in both cell types. These results indicate that the Tat protein acts similarly to FN in activating the Shc pathway, thus strengthening its ability to mimic extracellular matrix molecules. In addition, we also find that Shc/Grb2 signaling may contribute to the activation Ras and ERK in endothelial cells.
Figure 5.
Tat induces Shc phosphorylation and recruitment of its adaptor Grb2. Equal amounts of total proteins from both type of cells treated with 1 μg of Tat (middle panels) were immunoprecipitated with anti-Shc antibody followed by immunoblotting with either anti-phosphotyrosine or anti-Grb2 mAbs. Tat induces the Shc phosphorylation on tyrosine residues. (A and B) Shc associates with Grb2 within 5 min in HUVEC and EA-hy 926 cells. Intensities of the phosphorylated bands of Shc (arbitrary units, AU) were measured, and values are given on bottom of the top panels.
Control of Cell Cycle Progression by Tat
To examine whether Tat stimulation of the Ras/ERK pathway linked to Shc played a role in cell cycle progression, HUVEC and EA-hy 926 cells were plated on collagen type I and then synchronized in G0 by growth factor starvation for 24 and 36 h. The cells were then incubated for 24 h in a medium containing 25 ng/ml bFGF in the presence of either 10 μg/ml Tat, 25 μg/ml FN or BSA as control. Entry into the S phase was examined by BrdU incorporation and anti-BrdU staining. A large percentage of HUVEC incubated with Tat (18%) or FN (19%) compared with BSA (2%) entered the S phase during the 24 h assay (Figure 6A). Similar results were obtained with EA-hy 926 cells (Figure 6A). To further measure the relative ability of Tat versus soluble FN to enhance bFGF proliferation, we also studied whether Tat is able to induce Cyclin D1 expression. In fact, Cyclin D1 is known to be regulated at the transcriptional level by the Ras-ERK pathway and also by Rac and to be involved to the initial events necessary for progression through G1 and entry into the S phase (Mettouchi et al., 2001). Both HUVEC and EA-hy 926 cells were synchronized in G0 by growth factor deprivation and then incubated for 24 h in the above described medium, containing bFGF and 10 μg/ml Tat or 25 μg/ml FN. The cells were then lysed and analyzed for Cyclin D1 expression by immunoblotting. Figure 6B shows that Cyclin D1 accumulates to significant levels in both HUVEC and EA-hy 926 cells after the treatment with either Tat or FN. These results indicate that attachment and spreading on the extracellular matrix are not sufficient to induce progression of endothelial cells, either primary or immortalized, through G1 in response to mitogens. This process requires ligation of specific integrins, such as α5β1 and αvβ3, which are coupled to Ras signaling by Shc. In particular, Tat, similarly to FN, through its binding to these integrins is able to induce cell cycle progression.
Tat Induces Cell Cycle Progression through the Activation of ERK
To confirm that entry into the S phase determined by Tat is mediated by the activation of ERK, further experiments were performed using the inhibitor of ERK PD98059 on both HUVEC and EA-hy 926 cells. SB203580, a specific inhibitor of p38, was used as control. The treatment with PD98059 slightly affected the cell viability, nevertheless a consistent inhibitory effect on cell cycle progression was observed. In fact, fewer EA-hy 926 cells incubated with Tat in the presence of 25 μM PD98059 enter the S phase (61%) compared with those treated with Tat and DMSO (80%) or Tat and SB203580 (82%; Figure 7A, a-c). Comparable results have been obtained with HUVEC (Supplementary Figure 2).
The involvement of ERK1/2 activation in the cell proliferation induced by Tat was also studied by transfection of a kinase-dead mutant of mitogen-activated protein (MAP) kinase kinase 1 (MEK1), which is known to play a key role in the Ras-ERK pathway (Danen and Yamada, 2001), and blocks ERK1/2 activation in our cellular system (Supplementary Figure 3). To this extent EA-hy 926 cells were transfected with the kinase-dead MEK-K97M and then incubated for 24 h in a medium containing bFGF and Tat or FN. Entry into the S phase was monitored by BrdU incorporation, as described above. Only 8% of the cells transfected and treated with Tat-incorporated BrdU compared with 70% of the cells transfected with the control vector (Figure 7B, a and c). Similar results have been obtained in cells treated with FN (Figure 7B, b and d). The transfection with the kinase-dead MEK-K97M slightly affected either the cell viability and the proliferation, as observed previously after the treatment with the kinase inhibitors.
Taken together, these data indicate that Tat cooperates with bFGF in determining cell entry into the S phase and the process is specifically mediated by Tat's ability to induce the Ras/MAPK pathway. Consistent with this, the cells treated with Tat and a specific inhibitor of MAPK p38 activity still progress into the cell cycle after stimulation with Tat alone.
DISCUSSION
Previous studies by us and others have demonstrated the ability of HIV-1 Tat to cooperate with bFGF in promoting cell growth, motility, and angiogenesis and the development of KS-like lesions in mice. Tat has an important role in KS development and progression (Ensoli et al., 1994, 199b; Barillari et al., 1999a; Mitola et al., 2000). In the present report, we provide evidence that HIV-1 Tat binding on endothelial cells results in the activation of the Ras/ERK MAPK signaling pathway and enables the cells to progress through G1 in response to mitogens. In addition, we find that tyrosine phosphorylation of Shc and its association with Grb2 are upstream events accompanying the activation of Ras and ERK1/2 by Tat.
Cells require anchorage to extracellular matrix to proliferate. Integrin receptors activate signaling pathways that are responsible for the anchorage requirement. In particular, integrin-mediated cell adhesion can trigger activation of MAP kinases (Lin et al., 1997; Giancotti and Ruoslahti, 1999) and other protein kinases that are part of the consensus signaling pathway leading from receptor tyrosine kinases to Ras and then to a cytoplasmic kinase cascade comprising Raf, MEK1, MEK2, and MAP kinases (Lin et al., 1997). Integrin α5β1-mediated adhesion to FN is particularly efficient in the stimulation of cell cycle progression through the activation of the Ras and Rho family small GTPases (Danen and Yamada, 2001). In fact, a key role for Ras in the upstream events leading to ERK activation has been demonstrated in several cell types including endothelial, fibroblastic, and NK cells (Wary et al., 1996; Mainiero et al., 1998). Recent findings also show that the guanine exchange factor (GEF) Sos and its downstream target-effector Rac integrate signals from α5β1 integrins and growth factor receptors to promote biosynthesis of Cyclin D1 and progression through the G1 phase of the cell cycle, thus identifying a mechanism by which Rac is activated and controls the cell cycle machinery (Mettouchi et al., 2001). Our present results show that Tat ligation is able to induce activation of both Ras and Rac in endothelial cells. In addition, we demonstrate that Tat induces ERK1/2 activation via its RGD sequence, suggesting that Tat was binding to integrin receptors. This is in good agreement with the work of Ganju et al. (1998), showing that the RGD region of Tat induces MAP kinase activity in KS cells and with our previous studies demonstrating that KS and inflammatory cytokine-induced endothelial cell migration, invasion, growth, and adhesion are principally mediated by the binding of the Tat RGD region to αvβ3 and α5β1, which are highly expressed in AIDS-KS lesions (Ensoli et al., 1994; Barillari et al., 1999a, 1999b).
After transfection of Ras dominant negative or Ras- and Rac-specific siRNAs, which results in down-regulation of Ras function and Ras and Rac levels, respectively, we also observed a substantial inhibition of ERK1/2 phosphorylation by Tat only when Ras function and expression were silenced, thus suggesting the crucial role of Ras in the ERK pathway induced by Tat. By contrast, the activation of Rac by Tat does not correlate with ERK/MAPK activity. Consistent with this, it has been previously reported that the role of Rac in G1 cell cycle progression is principally related to its ability to organize the cytoskeleton and promote spreading, rather than to activate MAP kinases (Joneson et al., 1996; Lamarche et al., 1996; Westwick et al., 1997). Thus, it is likely that the activation of Rac by Tat could be linked with its ability to induce cell adhesion and motility (Barillari et al., 1993, 1999a; Albini et al., 1995). In fact, very recent findings indicate that Tat regulates endothelial cell actin cytoskeletal dynamics through oxidant production and the activation of the p21-activated kinase (Wu et al., 2004). This is the best characterized effector of Rac, which becomes activated by binding to GTP-loaded Rac and in this form, induced by extracellular matrix proteins, plays an important role on cell adhesion, spreading, and migration (Manser et al., 1994; Knaus et al., 1995; Lim et al., 1996; Price et al., 1998; Kiosses et al., 1999; del Pozo et al., 2000).
Integrin-induced Ras activation involves the Shc-mediated recruitment of the Grb-mSos complex to the plasma membrane to promote DNA synthesis (Wary et al., 1996; Mainiero et al., 1997, 1998; Wary et al., 1998). Accordingly, we demonstrate that binding of Tat results in tyrosine phosphorylation of the 52- and 46-kDa isoforms of Shc and association of Shc with Grb2 on both HUVEC and EA-hy 926 cells. In many nonimmortalized cells, the integrins that activate Shc signaling to ERK cooperate with growth factor receptors to promote cell proliferation (Wary et al., 1996; Mainiero et al., 1997; Pozzi et al., 1998). In addition to recruiting Shc, the α5β1 and αvβ3 integrins can form a physical complex with growth factor receptors and contribute to their ability to activate Ras-ERK signaling (Giancotti and Ruoslahti, 1999). In agreement with this, ShcA-/- fibroblasts display defective activation of ERK in response to both α5β1-mediated adhesion to FN and growth factor stimulation (Lai and Pawson, 2000).
Angiogenesis is a prerequisite for tumor growth and metastasis formation and a prominent feature of KS. Moreover, coordination of endothelial cell adhesion events with growth factor receptor activation regulates endothelial cell responses during angiogenesis (Eliceiri and Cheresh, 2001). Once released, the Tat protein is, at least in part, responsible for angiogenesis and KS development in HIV-infected individuals. In fact, our previous results indicated that the interaction of Tat with α5β1 and αvβ3 provides KS and endothelial cells with the adhesion signal that is required for their growth in response to mitogens, such as bFGF (Barillari et al., 1999b). To understand the mechanisms involved in these Tat effects, we show that engagement of specific integrins, such as α5β1 and αvβ3, by Tat promotes expression of Cyclin D1 and thus progression through G1 in response to bFGF, and this process is specifically mediated by the activation of ERK. In contrast, ligation of other integrins that bind, for example, type I collagen does not induce cell cycle progression even in the presence of a growth factor. These results are in good agreement with previous reports demonstrating that integrin α5β1-mediated cell adhesion to FN is particularly efficient in supporting growth factor-stimulated activation of MAPK and cell cycle progression (Symington, 1995; Danen et al., 2000; Kuwada and Li, 2000). In addition, other studies showed that β1 integrins are implicated in growth-factor-induced angiogenesis, and αvβ3-mediated endothelial cell migration and angiogenesis depend on the ligation state of α5β1 to FN (Kim et al., 2000b). Furthermore, antagonists of either α5β1 or αvβ3 block specifically bFGF-induced angiogenesis, suggesting that these two integrin receptors may regulate a similar angiogenic pathway (Friedlander et al., 1995; Kim et al., 2000a).
In conclusion, the findings presented here define the molecular mechanisms by which Tat is able to induce endothelial cell proliferation and, in particular, promotes cell progression through G1 in response to bFGF. This is relevant not only for defining the mechanisms characterizing the progression of AIDS-KS, but also these findings provide insight into the molecular basis of known antiangiogenic strategies, as well as into the design of novel anti-KS therapies.
Supplementary Material
Acknowledgments
We are grateful to Dr. Paolo Monini for helpful discussion, to Annamaria Carinci and Federica M. Regini for editorial assistance. This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) and the V Programma Nazionale di Ricerca sull'AIDS, Istituto Superiore di Sanità (ISS).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0717) on January 25, 2006.
Abbreviations used: AIDS-KS, acquired immune-deficiency syndrome-associated KS; bFGF, basic fibroblast growth factor; ERK, extracellular signal-related kinase; FN, fibronectin; HIV-1, human immunodeficiency virus-1; HUVEC, human umbilical endothelial cell; KS, Kaposi's sarcoma; MAPK, mitogen-activated protein kinase; RGD, arginine-glycine-aspartic acid.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Albini, A., Barillari, G., Benelli, R., Gallo, R. C., and Ensoli, B. (1995). Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc. Natl. Acad. Sci. USA 92, 4838-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini, A. et al. (1996). The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2, 1371-1375. [DOI] [PubMed] [Google Scholar]
- Asthagiri, A. R., Nelson, C. M., Horwitz, A. F., and Lauffenburger, D. A. (1999). Quantitative relationship among integrin-ligand binding, adhesion, and signaling via focal adhesion kinase and extracellular signal-regulated kinase 2. J. Biol. Chem. 274, 27119-27127. [DOI] [PubMed] [Google Scholar]
- Barillari, G., Buonaguro, L., Fiorelli, V., Hoffman, J., Michaels, F., Gallo, R. C., and Ensoli, B. (1992). Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi's sarcoma pathogenesis. J. Immunol. 149, 3727-3734. [PubMed] [Google Scholar]
- Barillari, G., Gendelman, R., Gallo, R. C., and Ensoli, B. (1993). The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 90, 7941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillari, G. et al. (1999a). The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the alpha5beta1 and alphavbeta3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 94, 663-672. [PubMed] [Google Scholar]
- Barillari, G. et al. (1999b). Inflammatory cytokines synergize with the HIV-1 Tat protein to promote angiogenesis and Kaposi's sarcoma via induction of basic fibroblast growth factor and the alpha v beta 3 integrin. J. Immunol. 163, 1929-1935. [PubMed] [Google Scholar]
- Chang, H. C., Samaniego, F., Nair, B. C., Buonaguro, L., and Ensoli, B. (1997). HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11, 1421-1431. [DOI] [PubMed] [Google Scholar]
- Danen, E. H., Sonneveld, P., Sonnenberg, A., and Yamada, K. M. (2000). Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to fibronectin supports growth factor-stimulated cell cycle progression. J. Cell Biol. 151, 1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen, E. H., and Yamada, K. M. (2001). Fibronectin, integrins, and growth control. J. Cell Physiol. 189, 1-13. [DOI] [PubMed] [Google Scholar]
- del Pozo, M. A., Price, L. S., Alderson, N. B., Ren, X. D., and Schwartz, M. A. (2000). Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19, 2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykxhoorn, D. M., Novina, C. D., and Sharp, P. A. (2003). Killing the messenger: short RNAs that silence gene expression. Nat. Rev. Mol. Cell Biol. 4, 457467. [DOI] [PubMed] [Google Scholar]
- Edgell, C. J., McDonald, C. C., and Graham, J. B. (1983). Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 80, 3734-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri, B. P., and Cheresh, D. A. (2001). Adhesion events in angiogenesis. Curr. Opin. Cell Biol. 13, 563-568. [DOI] [PubMed] [Google Scholar]
- Eliceiri, B. P., Klemke, R., Stromblad, S., and Cheresh, D. A. (1998). Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J. Cell Biol. 140, 1255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli, B., Barillari, G., Salahuddin, S. Z., Gallo, R. C., and Wong-Staal, F. (1990). Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345, 84-86. [DOI] [PubMed] [Google Scholar]
- Ensoli, B., Buonaguro, L., Barillari, G., Fiorelli, V., Gendelman, R., Morgan, R. A., Wingfield, P., and Gallo, R. C. (1993). Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67, 277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli, B., Gendelman, R., Markham, P., Fiorelli, V., Colombini, S., Raffeld, M., Cafaro, A., Chang, H. K., Brady, J. N., and Gallo, R. C. (1994). Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature 371, 674-680. [DOI] [PubMed] [Google Scholar]
- Even-Ram, S., and Yamada, K. M. (2005). Cell migration in 3D matrix. Curr. Opin. Cell Biol. 17, 524-532. [DOI] [PubMed] [Google Scholar]
- Fiorelli, V., Barillari, G., Toschi, E., Sgadari, C., Monini, P., Sturzl, M., and Ensoli, B. (1999). IFN-gamma induces endothelial cells to proliferate and to invade the extracellular matrix in response to the HIV-1 Tat protein: implications for AIDS-Kaposi's sarcoma pathogenesis. J. Immunol. 162, 1165-1170. [PubMed] [Google Scholar]
- Fiorelli, V., Gendelman, R., Samaniego, F., Markham, P. D., and Ensoli, B. (1995). Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi's sarcoma spindle cells. J. Clin. Invest. 95, 1723-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli, V. et al. (1998). gamma-Interferon produced by CD8+ T cells infiltrating Kaposi's sarcoma induces spindle cells with angiogenic phenotype and synergy with human immunodeficiency virus-1 Tat protein: an immune response to human herpesvirus-8 infection? Blood 91, 956-967. [PubMed] [Google Scholar]
- Friedlander, M., Brooks, P. C., Shaffer, R. W., Kincaid, C. M., Varner, J. A., and Cheresh, D. A. (1995). Definition of two angiogenic pathways by distinct alpha v integrins. Science 270, 1500-1502. [DOI] [PubMed] [Google Scholar]
- Ganju, R. K., Munshi, N., Nair, B. C., Liu, Z. Y., Gill, P., and Groopman, J. E. (1998). Human immunodeficiency virus tat modulates the Flk-1/KDR receptor, mitogen-activated protein kinases, and components of focal adhesion in Kaposi's sarcoma cells. J. Virol. 72, 6131-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti, F. G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Joneson, T., McDonough, M., Bar-Sagi, D., and Van Aelst, L. (1996). RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science 274, 1374-1376. [DOI] [PubMed] [Google Scholar]
- Kim, S., Bell, K., Mousa, S. A., and Varner, J. A. (2000a). Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am. J. Pathol. 156, 1345-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., Harris, M., and Varner, J. A. (2000b). Regulation of integrin alpha vbeta 3-mediated endothelial cell migration and angiogenesis by integrin alpha5beta1 and protein kinase A. J. Biol. Chem. 275, 33920-33928. [DOI] [PubMed] [Google Scholar]
- Kinbara, K., Goldfinger, L. E., Hansen, M., Chou, F. L., and Ginsberg, M. H. (2003). Ras GTPases: integrins' friends or foes? Nat. Rev. Mol. Cell Biol. 4, 767-776. [DOI] [PubMed] [Google Scholar]
- Kiosses, W. B., Daniels, R. H., Otey, C., Bokoch, G. M., and Schwartz, M. A. (1999). A role for p21-activated kinase in endothelial cell migration. J. Cell Biol. 147, 831-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus, U. G., Morris, S., Dong, H. J., Chernoff, J., and Bokoch, G. M. (1995). Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science 269, 221-223. [DOI] [PubMed] [Google Scholar]
- Kumar, A., Manna, S. K., Dhawan, S., and Aggarwal, B. B. (1998). HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J. Immunol. 161, 776-781. [PubMed] [Google Scholar]
- Kuwada, S. K., and Li, X. (2000). Integrin alpha5/beta1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol. Biol. Cell 11, 2485-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, K. M., and Pawson, T. (2000). The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 14, 1132-1145. [PMC free article] [PubMed] [Google Scholar]
- Lamarche, N., Tapon, N., Stowers, L., Burbelo, P. D., Aspenstrom, P., Bridges, T., Chant, J., and Hall, A. (1996). Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87, 519-529. [DOI] [PubMed] [Google Scholar]
- Lim, L., Manser, E., Leung, T., and Hall, C. (1996). Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. Eur. J. Biochem. 242, 171-185. [DOI] [PubMed] [Google Scholar]
- Lin, T. H., Chen, Q., Howe, A., and Juliano, R. L. (1997). Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J. Biol. Chem. 272, 8849-8852. [PubMed] [Google Scholar]
- Mainiero, F., Gismondi, A., Soriani, A., Cippitelli, M., Palmieri, G., Jacobelli, J., Piccoli, M., Frati, L., and Santoni, A. (1998). Integrin-mediated ras-extracellular regulated kinase (ERK) signaling regulates interferon gamma production in human natural killer cells. J. Exp. Med. 188, 1267-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero, F., Murgia, C., Wary, K. K., Curatola, A. M., Pepe, A., Blumemberg, M., Westwick, J. K., Der, C. J., and Giancotti, F. G. (1997). The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 16, 2365-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero, F., Pepe, A., Wary, K. K., Spinardi, L., Mohammadi, M., Schlessinger, J., and Giancotti, F. G. (1995). Signal transduction by the alpha 6 beta 4 integrin: distinct beta 4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 14, 4470-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser, E., Leung, T., Salihuddin, H., Zhao, Z. S., and Lim, L. (1994). A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40-46. [DOI] [PubMed] [Google Scholar]
- Mettouchi, A., Klein, S., Guo, W., Lopez-Lago, M., Lemichez, E., Westwick, J. K., and Giancotti, F. G. (2001). Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol. Cell 8, 115-127. [DOI] [PubMed] [Google Scholar]
- Milani, D., Mazzoni, M., Borgatti, P., Zauli, G., Cantley, L., and Capitani, S. (1996). Extracellular human immunodeficiency virus type-1 Tat protein activates phosphatidylinositol 3-kinase in PC12 neuronal cells. J. Biol. Chem. 271, 22961-22964. [DOI] [PubMed] [Google Scholar]
- Milani, D., Mazzoni, M., Zauli, G., Mischiati, C., Gibellini, D., Giacca, M., and Capitani, S. (1998). HIV-1 Tat induces tyrosine phosphorylation of p125FAK and its association with phosphoinositide 3-kinase in PC12 cells. AIDS 12, 1275-1284. [DOI] [PubMed] [Google Scholar]
- Mischiati, C., Pironi, F., Milani, D., Giacca, M., Mirandola, P., Capitani, S., and Zauli, G. (1999). Extracellular HIV-1 Tat protein differentially activates the JNK and ERK/MAPK pathways in CD4 T cells. AIDS 13, 1637-1645. [DOI] [PubMed] [Google Scholar]
- Mitola, S., Soldi, R., Zanon, I., Barra, L., Gutierrez, M. I., Berkhout, B., Giacca, M., and Bussolino, F. (2000). Identification of specific molecular structures of human immunodeficiency virus type 1 Tat relevant for its biological effects on vascular endothelial cells. J. Virol. 74, 344-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi, A., Wary, K. K., Giancotti, F. G., and Gardner, H. A. (1998). Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J. Cell Biol. 142, 587-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, L. S., Leng, J., Schwartz, M. A., and Bokoch, G. M. (1998). Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9, 1863-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnati, M., Urbinati, C., Musulin, B., Ribatti, D., Albini, A., Noonan, D., Marchisone, C., Waltenberger, J., and Presta, M. (2001). Activation of endothelial cell mitogen activated protein kinase ERK(1/2) by extracellular HIV-1 Tat protein. Endothelium 8, 65-74. [DOI] [PubMed] [Google Scholar]
- Samaniego, F., Markham, P. D., Gallo, R. C., and Ensoli, B. (1995). Inflammatory cytokines induce AIDS-Kaposi's sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi's sarcoma-like lesion formation in nude mice. J. Immunol. 154, 3582-3592. [PubMed] [Google Scholar]
- Samaniego, F., Markham, P. D., Gendelman, R., Gallo, R. C., and Ensoli, B. (1997). Inflammatory cytokines induce endothelial cells to produce and release basic fibroblast growth factor and to promote Kaposi's sarcoma-like lesions in nude mice. J. Immunol. 158, 1887-1894. [PubMed] [Google Scholar]
- Samaniego, F., Markham, P. D., Gendelman, R., Watanabe, Y., Kao, V., Kowalski, K., Sonnabend, J. A., Pintus, A., Gallo, R. C., and Ensoli, B. (1998). Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 152, 1433-1443. [PMC free article] [PubMed] [Google Scholar]
- Symington, B. E. (1995). Growth signalling through the alpha 5 beta 1 fibronectin receptor. Biochem. Biophys. Res. Commun. 208, 126-134. [DOI] [PubMed] [Google Scholar]
- Toschi, E. et al. (2001). Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol. Biol. Cell 12, 2934-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary, K. K., Mainiero, F., Isakoff, S. J., Marcantonio, E. E., and Giancotti, F. G. (1996). The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87, 733-743. [DOI] [PubMed] [Google Scholar]
- Wary, K. K., Mariotti, A., Zurzolo, C., and Giancotti, F. G. (1998). A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625-634. [DOI] [PubMed] [Google Scholar]
- Westwick, J. K., Lambert, Q. T., Clark, G. J., Symons, M., Van Aelst, L., Pestell, R. G., and Der, C. J. (1997). Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol. Cell. Biol. 17, 1324-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, R. F., Gu, Y., Xu, Y. C., Mitola, S., Bussolino, F., and Terada, L. S. (2004). Human immunodeficiency virus type 1 Tat regulates endothelial cell actin cytoskeletal dynamics through PAK1 activation and oxidant production. J. Virol. 78, 779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.