Abstract
Glycogen synthase kinase-3 (GSK-3) is a multifunctional serine/threonine kinase that is usually inactivated by serine phosphorylation in response to extracellular cues. However, GSK-3 can also be activated by tyrosine phosphorylation, but little is known about the upstream signaling events and tyrosine kinase(s) involved. Here we describe a G protein signaling pathway leading to GSK-3 activation during lysophosphatidic acid (LPA)-induced neurite retraction. Using neuronal cells expressing the LPA1 receptor, we show that LPA1 mediates tyrosine phosphorylation and activation of GSK-3 with subsequent phosphorylation of the microtubule-associated protein tau via the Gi-linked PIP2 hydrolysis-Ca2+ mobilization pathway. LPA concomitantly activates the Ca2+-dependent tyrosine kinase Pyk2, which is detected in a complex with GSK-3β. Inactivation or knockdown of Pyk2 inhibits LPA-induced (but not basal) tyrosine phosphorylation of GSK-3 and partially inhibits LPA-induced neurite retraction, similar to what is observed following GSK-3 inhibition. Thus, Pyk2 mediates LPA1-induced activation of GSK-3 and subsequent phosphorylation of microtubule-associated proteins. Pyk2-mediated GSK-3 activation is initiated by PIP2 hydrolysis and may serve to destabilize microtubules during actomyosin-driven neurite retraction.
INTRODUCTION
Glycogen synthase kinase-3 (GSK-3) is a ubiquitous serine/threonine kinase that regulates numerous cellular processes, ranging from glycogen metabolism to morphogenesis and cell proliferation. Dysregulation of GSK-3 activity has been implicated in several human diseases, including diabetes, Alzheimer's disease, and cancer (Frame and Cohen, 2001; Doble and Woodgett, 2003; Jope and Johnson, 2004). Mammalian GSK-3 exists as two isoforms encoded by distinct genes, GSK-3α (51 kDa) and GSK-3β (47 kDa; Woodgett, 1990), with two splice variants of GSK-3β (Mukai et al., 2002; Schaffer et al., 2003). Highest expression of GSK-3 is observed in developing brain, where its expression correlates with the period of active neurite remodeling (Woodgett, 1990; Takahashi et al., 1994; Leroy and Brion, 1999). In accordance with this, studies on cultured neuronal cells point to an important role of GSK-3 in regulating neurite morphology (Eickholt et al., 2002; Sayas et al., 2002a; Zhou et al., 2004; Yoshimura et al., 2005; Jiang et al., 2005): inactivation and activation of GSK-3 promote neurite outgrowth and withdrawal, respectively, the key processes in nervous system development and plasticity. The ability of GSK-3 to regulate neuronal architecture is thought to rely on its ability to phosphorylate microtubule-binding proteins, particularly the neuron-specific proteins tau (Hanger et al., 1992; Wagner et al., 1996), MAP1B (Trivedi et al., 2005) and CRMP-2 (Yoshimura et al., 2005), and the widely expressed adenomatous polyposis coli protein (APC; Zhou et al., 2004). Phosphorylation of these proteins by GSK-3β promotes microtubule disassembly and destabilization, which inhibits or repels axon growth (Zhou and Snider, 2005). Importantly, hyperphosphorylated tau is a major component of the neurofibrillary tangles detected in certain neurodegenerative diseases including Alzheimer's disease (Grimes and Jope, 2001; Drewes, 2004), which provides a strong rationale for developing selective GSK-3 inhibitors for the treatment of such neuropathologies (Cohen and Goedert, 2004).
The activity of GSK-3 is controlled by phosphorylation and interaction with inhibitory proteins. Unlike other protein kinases, GSK-3 is constitutively active under resting conditions and is inactivated by extracellular signals through phosphorylation of an N-terminal serine residue, Ser-9 in GSK-3β and Ser-21 in GSK-3α. Various kinases have been implicated in mediating serine phosphorylation and inactivation of GSK-3, including PI3-kinase (PI3K)-regulated Akt/PKB, protein kinase A and protein kinase C (PKC; Cross et al., 1995; Fang et al., 2000, 2002). GSK-3 also plays a central role in the canonical Wnt pathway, in which the enzyme is displaced from a multiprotein complex and thereby unable to phosphorylate its substrates such as β-catenin (Doble and Woodgett, 2003).
In opposition to inhibitory serine phosphorylation, GSK-3 activity is increased by phosphorylation of a tyrosine residue, Tyr-216 in GSK-3β and Tyr-279 in GSK-3α, located in the kinase domain. This phosphotyrosine is important for activity because its dephosphorylation diminishes activity (Hughes et al., 1993; Wang et al., 1994), but the mechanism responsible for tyrosine phosphorylation of GSK-3 remains unclear. In Dictyostelium discoideum, the tyrosine kinase ZAK1 mediates GSK-3 phosphorylation and activation in response to cAMP (Kim et al., 1999). In mammalian cells, the tyrosine kinases Fyn, Pyk2, and Csk have been implicated in phosphorylating GSK-3 (Lesort et al., 1999; Hartigan et al., 2001; Fan et al., 2003), but some of these claims have been questioned (Cole et al., 2004).
In neuronal cells, GSK-3 is tyrosine phosphorylated and activated during neurite retraction induced by the serum-borne lipid mediator lysophosphatidic acid (LPA; Sayas et al., 1999, 2002b), but how LPA activates GSK-3 is unclear. LPA acts on at least four distinct G protein-coupled receptors (GPCRs), termed LPA1-4 (Chun et al., 2002; Noguchi et al., 2003), that signal via multiple G proteins, including Gq/11, Gi/o, and G12/13, to induce a great diversity of cellular responses (Moolenaar et al., 2004). LPA-induced neurite retraction is primarily driven by actomyosin-based contractile forces initiated by Gα12/13-linked activation of RhoA and its downstream effector Rho-kinase (ROCK; Jalink et al., 1994; Hirose et al., 1998; Kranenburg et al., 1999). Activated GSK-3 may contribute to optimal neurite retraction by phosphorylating microtubule-binding proteins leading to microtubule destabilization. In the present study, we set out to identify the G protein-effector pathway and the tyrosine kinase that mediates phosphorylation and activation of GSK-3 in neuronal cells after stimulation of the prototypic LPA1 receptor. We show that GSK-3 is tyrosine phosphorylated by the Ca2+-sensitive tyrosine kinase Pyk2 as a direct consequence of phospholipase C activation.
MATERIALS AND METHODS
Cells and Materials
B103, B103-LPA1, Neuro2A, PC12, and SH-SY5Y cells were routinely grown in DMEM containing 10% fetal calf serum. The generation of B103-LPA1 cells has been described previously (Van Leeuwen et al., 2003). Neurite outgrowth was induced by exposing the cells to serum-free medium for >18 h or, in case of SH-SY5Y cells, Neurobasal medium containing B-27 supplement and 1 mM db-cAMP for 72 h (Sayas et al., 1999). Chemicals and reagents were obtained from the following sources: 1-oleoyl-LPA, Sigma (Zwijndrecht, The Netherlands); BAPTA-AM, tyrphostin A9, PP2, and AG-1296, Calbiochem (La Jolla, CA); SB-216763 and SB-415286, Tocris (Ballwin, MO); pertussis toxin, List Biological Laboratories (Campbell, CA). The following antibodies were used: anti-GSK-3α/β monoclonal antibody (mAb) and rabbit anti-phospho-GSK-3α/β (pTyr279/216), Biosource (Camarillo, CA) and Sigma; rabbit phospho-GSK-3α/β (pSer21/9), Cell Signaling (Beverly, MA); anti-GSK-3β mAb, BD Transduction Laboratories (Lexington, KY). Monoclonal 7.51 against total tau was a gift from J. Avila (Centro de Biologia Molecular, Madrid, Spain). Antibody PHF-1 against phosphorylated tau (Ser396/404) was supplied by P. Davies (Albert Einstein College, New York). Rabbit anti-pTyr402-Pyk2 was obtained from Biosource and mAb against total Pyk2 was from BD Transduction Laboratories. Monoclonal anti-phosphotyrosine antibody 4G10 was from Upstate Biotechnology (Lake Placid, NY), monoclonal anti-Flag M2 from Sigma and polyclonal anti-GFP antibody from J. Neefjes (NKI, Amsterdam, The Netherlands).
Cell Morphology
Serum-starved cells in 35-mm dishes were placed in a heat chamber (37°C) with a constant CO2 infusion. Phase-contrast and corresponding fluorescence images were taken on a Zeiss inverted microscope (Thornwood, NY) equipped with a CCD camera. Neurites were defined as processes longer than one cell body diameter.
Immunoblotting
Total cell lysates were prepared by washing the cells with phosphate-buffered saline (PBS) followed by resuspension in lysis buffer containing 1% SDS, 1 mM EDTA, 1 mM EGTA, and 25 mM Tris (pH 7.5). Lysates were boiled for 10 min., spun down, and sonicated for 15 s. Proteins (25-50 μg) were separated by SDS-PAGE and transferred to nitrocellulose filters. The filters were blocked with 10% nonfat milk powder or 5% BSA in Tris-buffered saline (TBS)-0.1% Tween 20 (TBS-T), and subsequently incubated with primary antibodies overnight (4°C) followed by incubation with the corresponding peroxidase-conjugated secondary antibody (DAKO, Carpinteria, CA) for 1 h. Immunoreactivity was visualized by enhanced chemiluminescence detection (Amersham). Densitometric analysis was performed using TINA 2.0 software.
GSK-3 In Vitro Kinase Assay
To assay GSK-3 activity in cell extracts, control and LPA-treated B103-LPA1 cells were lysed in a buffer containing 100 mM NaCl, 100 mM NaF, 1 mM sodium orthovanadate, 5 mM EDTA, 100 nM okadaic acid, 20 mM HEPES (pH 7.4), 1% Triton X-100 and protease inhibitors. Lysates were centrifuged at 14000 rpm for 15 min at 4°C to obtain the soluble fraction, which was used as a source of GSK-3 activity. Soluble cell extracts (7 μg) were incubated in a buffer containing 25 mM Tris (pH 7.5), 1 mM DTT, 10 mM MgCl2, and the GSK-3-specific eIF2B-based substrate peptide 2B-SP (0.75 mg/ml; Welsh et al., 1997) in the presence of [γ-32P]ATP. The reaction was stopped after 1 h by spotting aliquots on P81 phosphocellulose paper followed by scintillation counting. Assays were performed in the presence or absence of LiCl (20 mM; Sayas et al., 1999). The difference between the kinase activity in the presence or absence of LiCl was considered a measure of GSK-3 activity. Activity values were normalized with respect to GSK-3 expression levels.
Transfection and Immunoprecipitation
B103-LPA1 and Neuro2A cells were transfected using Lipofectamine Plus reagents (Invitrogen, Carlsbad, CA) or Fugene (Roche), respectively. The following constructs were used: GFP-tagged versions of human wild-type Pyk2 and kinase-dead Pyk2 (Pyk2 K457A; provided by Drs. Sancho and Sanchez-Madrid, Hospital de la Princesa, Madrid, Spain); HA-tagged human GSK-3β (a gift from J. Woodgett, The Ontario Cancer Institute, Toronto, Canada); Myc-tagged mouse GSK-3β and kinase-inactive GSK-3β(R85) (KI-GSK-3β-Myc; from F. Wandosell, Centro de Biologia Molecular “Severo Ochoa,” Madrid, Spain) and Myc-tagged rat Pyk2 (a gift from H. Earp, University of North Carolina at Chapel Hill, North Carolina). Because B103 transfection efficiency is low, we cotransfected a plasmid bearing puromycin resistance. Transfected cells were maintained in the presence of puromycin (1 μg/ml) for 30-48 h to enrich the population of transfected cells. Human SH-SY5Y neuroblastoma cells were transfected with a GFP-tagged human Pyk2 construct (gift from Drs. Sancho and Sanchez Madrid, Hospital de La Princesa) or pEGFP (Clontech), using Lipofectamine Plus reagents (Invitrogen). Cells were then selected for 2 wk in the presence of G-418 (GIBCO, Invtrogen Cell Culture). Selected cells were grown for 2 more weeks to obtain stable SH-SY5Y-GFP and SH-SY5Y-Pyk2-GFP cell lines. SH-SY5Y cells were differentiated as described (Sayas et al., 1999). For immunoprecipitation, cells were washed twice with PBS and lysed in a buffer containing 50 mM Tris (pH 7.5), 1 mM EDTA, 150 mM NaCl, 10 mM NaF, 1% NP-40, 2 mM sodium orthovanadate, and protease inhibitors. Lysates were centrifuged at 14,000 rpm, precleared, and incubated with the appropriate primary antibodies overnight at 4°C. Thereafter, protein A or G was added to the lysates and incubated at 4°C for 1 h. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting.
Immunofluorescence and Quantification of Cell Contraction
B103-LPA1 cells were cotransfected with either pcDNA3-Myc and GFP or KI-GSK-3β-Myc and GFP in a 3:1 ratio. Cells were treated with LPA for 15-30 min or left untreated. After treatments, cell cultures were fixed with PBS containing 4% (wt/vol) paraformaldehyde for 20 min. After several washes with PBS, the cells were preincubated in PBS, containing 0.1% Triton X-100 and 3% BSA for 30 min. Cells were then incubated overnight at 4°C with mAb anti-Flag M2. After washing, cultures were incubated with the appropriate secondary antibody, conjugated to Alexa-568 (Molecular Probes, Eugene, OR) for 45 min. After washing, coverslips were mounted with Vectashield (Vector Laboratories, Burlingame, CA). Cells were visualized with a Leica fluorescence microscope. Green cells with and without neurites were counted in each case. Pictures were taken with a CCD camera.
Pyk2 RNA Interference
Four different Pyk2 small interfering RNA (siRNA)-targeting vectors (pS-GFP-Pyk2) were based on four 19-mer sequences present in the coding sequence of rat Pyk2: 1) 5′-tgcacagtgcagacagaga-3′; 2) 5′-ctcattcaagggtggaaca-3′; 3) 5′-gatgtagttcttaaccgca-3′; 4) 5′-gatgcttggacccgatggt-3′. Blast search revealed no off-sequence targeting. 64-mer synthetic oligonucleotides for cloning into pSuperGFP (pS-GFP) were synthesized, annealed, and ligated into pS-GFP, which includes GFP under control of the pGK promoter (Brummelkamp et al., 2002; Mulder et al., 2004). Thus, detection of cells expressing the siRNAs was possible by searching for GFP-positive cells. Of the four different targeting constructs tested, pS-GFP-Pyk2-3 and pS-GFP-Pyk2-4 were most efficient in down-regulating Pyk2 mRNA. Adenoviral pAS constructs were designed as follows: pS-GFP-Pyk2-3 or pS-GFP were digested and the insert containing the RNAi targeting sequence and promotor was ligated into pENTR1A of the virapower adenoviral expression system (Invitrogen).
Measurement of PIP2 and Ca2+ Mobilization
Agonist-induced hydrolysis of phosphatidylinositol (4,5)-bisphosphate (PIP2) was monitored in real-time by fluorescence resonance energy transfer (FRET) between pleckstrin homology (PH) domains of PLC-δ1 fused to cyan and yellow fluorescent proteins (CFP and YFP), as described previously (van der Wal et al., 2001). PH-CFP and PH-YFP chimeric constructs were transiently transfected into B103-LPA1 cells at a 1:1 M ratio. After 24 h, cells were transferred to an inverted epifluorescence microscope and assayed for FRET by simultaneously monitoring the emission of CFP (475 nm) and YFP (530 nm), while exciting CFP at 425 nm. After hydrolysis of PIP2, the PH domains rapidly dissociate from the plasma membrane and the FRET signal decreases (van der Wal et al., 2001). Ca2+ mobilization was measured by loading the cells with the Ca2+-sensitive fluorescent dye Indo-1/AM (Molecular Probes) and recording fluorescence intensity (excitation 355 nm, emission 405 nm), as described previously (Jalink et al., 1995).
RESULTS
GSK-3 Phosphorylation Mediated by LPA1
Most cultured cells coexpress at least two distinct LPA receptors, which hampers the dissection of receptor-specific signaling pathways. LPA1 is the predominant receptor in the nervous system, where it is spatially and temporarily regulated throughout development (Fukushima et al., 2001). We therefore used B103-LPA1 neuronal cells, which stably express LPA1 at relatively low levels (Van Leeuwen et al., 2003). Removal of serum from these cells results in the formation of neurites, which rapidly retract in response to LPA and other GPCR agonists such as sphingosine 1-phosphate (S1P) and thrombin (Van Leeuwen et al., 2003 and unpublished data).
GSK-3 phosphorylation was examined using phospho-specific antibodies, notably anti-phosphotyrosine-GSK-3(α/β) (pTyr279/216) and anti-phosphoserine-GSK-3(α/β) (pSer21/9). Monoclonal anti-GSK-3(α/β) was used to detect total GSK-3. Treatment of B103-LPA1 cells with 1-oleoyl-LPA (5 μM) induced a rapid, transient increase in tyrosine phosphorylation of GSK-3 above basal levels, reaching a maximum at 2-5 min and accompanying cytoskeletal contraction (Figure 1A, top panel; see also Figure 9A). As expected, LPA receptor-deficient B103 cells failed to respond to LPA (unpublished data). Similar to LPA, other “collapsing” agonists such as S1P and thrombin receptor-activating peptide (TRP) also induced GSK-3 tyrosine phosphorylation (Figure 1B).
Figure 1.
LPA-induced phosphorylation of GSK-3 in B103-LPA1 cells. (A) Cells were treated with LPA (5 μM) for the indicated time periods. Cells were lysed and GSK-3 phosphorylation was analyzed using phospho-specific antibodies (anti-pSer21/9 or anti-pTyr279/216 GSK-3α/β). Membranes were reprobed with an antibody against total GSK-3α/β to show similar loading levels. Middle panel, the ratio between tyrosine-phosphorylated GSK-3α/β and total GSK-3α/β, as determined by densitometry. Bottom panel, the ratio GSK-3α-PY versus total GSK-3α (left) and GSK-3β-PY versus total GSK-3β (right; n ≥ 6; error bars, SD). (B) Immunoblot analysis of total GSK-3 tyrosine phosphorylation in cells treated for 5 min with either LPA (5 μM), thrombin receptor-activating peptide (TRP, 100 μM) or S1P (200 nM). (C) Cells were treated with sodium pervanadate (PV) for 5 min and GSK-3 phosphotyrosine was analyzed by immunoblotting as in A. (D) Immunoblot analysis of GSK-3α/β serine phosphorylation in lysates from cells treated with LPA (5 μM) or with insulin (1 μM) for the indicated time points. Bar graph shows the ratio between the densitometric values of phospho-serine GSK-3α/β and total GSK-3α/β; error bars, SD (n > 3).
Figure 9.
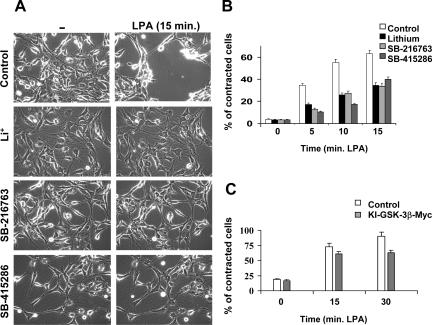
Effect of GSK-3 inhibition on LPA1-mediated neurite retraction. (A) Typical examples of contractile responses to LPA (5 μM; 15 min) in the presence or absence of three different GSK-3 inhibitors, LiCl (20 mM, 5 h), SB-216763 (25 μM, 20 min), and SB-415286 (40 μM, 20 min). Pictures of control and LPA-treated cells were taken from the same microscopic field. (B) Analysis of LPA-induced neurite retraction/cell contraction in serum-starved B103-LPA1 cells and the effect of the three indicated GSK-3 inhibitors. Data represent the means (±SD) of five different experiments. (C) Quantification of cell contraction in B103-LPA1 cells cotransfected with either pcDNA3-Myc (Control) or kinase-dead KI-GSK-3β-Myc together with GFP. Cells were treated with LPA (5 μM) for 15 and 30 min. Data represent the means (±SD) of three different experiments. (Note that the number of contracted cells at time zero is ∼10% larger than in B, probably because of some toxicity of Lipofectamine.)
Tyrosine phosphorylation was detected in GSK-3β rather than in GSK-3α, at least with the antibodies used (from two distinct commercial sources): densitometric analysis showed an ∼1.8-fold increase in GSK-3β phosphotyrosine (Figure 1A, bottom panels; a minor increase in GSK-3α, detected in some experiments, was barely significant). For convenience, we combined the α- and β-isoform phosphotyrosine levels in our further analysis of GSK-3. As can be seen in Figure 1, A-C, a third band running below GSK-3β was consistently recognized by anti-phosphotyrosine-GSK-3 antibody; its appearance closely correlated with the increase in tyrosine phosphorylation of GSK-3β. Treatment of the cells with the tyrosine phosphatase inhibitor pervanadate markedly enhanced the intensity of all three bands (Figure 1C), indicating that the third band, similar to GSK-3α/β, is a target for tyrosine kinases. Prolonged exposure of the blots probed with antibodies against total GSK-3α/β likewise revealed an additional band running just below GSK-3β (unpublished data). We presume this band represents the GSK-3β splice variant that lacks exon 10 (encoding 33 residues; Schaffer et al., 2003), as it differs from “nonspliced” GSK-3β by the predicted molecular size.
LPA also induced serine phosphorylation of GSK-3 (again, particularly the β isoform), with kinetics similar to those of tyrosine phophorylation (Figure 1B). Of note, serine phosphorylation of GSK-3 induced by LPA was weaker than that induced by insulin (Figure 1D), the classic agonist that phosphorylates GSK-3 via the PI3K-Akt/PKB pathway to inhibit its activity (Cross et al., 1995). Pharmacological inhibitor studies suggested that LPA-induced serine phosphorylation of GSK-3 involves the wortmannin-sensitive PI3K-Akt/PKB pathway as well as PKC (unpublished data), but we did not examine the responsible signaling pathways in further detail. The use of pharmacological PI3K and PKC inhibitors allowed an independent confirmation that the anti-GSK-3 phosphotyrosine antibodies were specific in that they did not recognize serine-phosphorylated GSK-3 (unpublished data). Taken together, these results indicate that LPA1 receptor stimulation leads to GSK-3 phosphorylation on both tyrosine and serine residues.
GSK-3 Activation and Tau Phosphorylation
Serine phosphorylation of GSK-3 inhibits its catalytic activity, whereas tyrosine phosphorylation has the opposite effect (Wang et al., 1994; Doble and Woodgett, 2003). To determine how dual Tyr/Ser phosphorylation of GSK-3 affects its enzymatic activity, we performed in vitro kinase assays using a GSK-3-specific substrate peptide. As shown in Figure 2A, LPA induces a net increase in GSK-3 activity, which reaches a maximum at 2 min and returns to basal levels after ∼10 min of LPA addition (Figure 2A). Thus, the net effect of dual Tyr/Ser phosphorylation of GSK-3 by LPA1 is a transient increase in activity. Our further experiments focused on GSK-3 tyrosine phosphorylation and activation, as serine phosphorylation was beyond the scope of the present study.
Figure 2.

LPA-induced GSK-3 activation and tau phosphorylation in B103-LPA1 cells. (A) GSK-3 kinase activity after addition of LPA (5 μM). Kinase activity at each time point was normalized with respect to the total amount of GSK-3α/β present in each cell lysate. Nonstimulated controls were defined as 100 “relative units” (r.u.). Data represent the mean (±SD) of three independent experiments. (B) Tau phosphorylation in response to LPA (5 μM), detected by anti-phospho-tau antibody PHF-1. (C) Effect of the GSK-3 inhibitor SB-415286 (40 μM, added 30 min before LPA). Antibody 7.51 against total tau was used for the loading control.
One neuron-specific target of GSK-3 is the microtubule-associated protein tau (Hanger et al., 1992). We examined the phosphorylation state of tau using phospho-specific antibody PHF-1 (anti-pSer396/404 tau) together with monoclonal 7.51 against total tau. Consistent with tau being a downstream target of GSK-3, LPA induced a transient increase in tau phosphorylation (Figure 2B), which was completely inhibited by the GSK-3 inhibitor SB-415286 (40 μM) and two other structurally unrelated inhibitors, SB-216763 (25 μM) and LiCl (20 mM; Cohen and Goedert, 2004; Figure 2C and unpublished data).
GSK-3 Tyrosine Phosphorylation via Gi-mediated PIP2 Hydrolysis and Ca2+ Mobilization
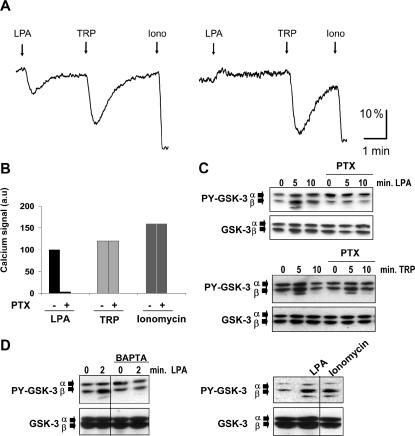
We next explored the LPA1-linked signaling pathway that leads to GSK-3 tyrosine phosphorylation in B103-LPA1 cells. Because intracellular Ca2+ has been implicated in GSK-3 activation (Hartigan and Johnson, 1999) and LPA is a potent Ca2+-mobilizing agonist for numerous cell types (Jalink et al., 1990), we analyzed phospholipase C (PLC) activation following LPA1 stimulation, using thrombin receptor-activating peptide (TRP) as a control. The levels of phosphatidylinositol 4,5-bisphosphate (PIP2) were monitored in real-time using FRET between the PH domains of PLC-δ1 fused to CFP and YFP, respectively (van der Wal et al., 2001). Figure 3A illustrates that LPA and TRP induced a rapid, transient decrease in PIP2 levels accompanied by a rise in cytosolic free Ca2+ (Figure 3B). Pretreatment of the cells with pertussis toxin (PTX), a selective inhibitor of Gi, largely abrogated LPA-induced PIP2 hydrolysis, Ca2+ mobilization and GSK-3 tyrosine phosphorylation, without affecting the responses to TRP (Figure 3C). Chelation of intracellular free Ca2+ using cell-permeable BAPTA-AM blocked LPA-induced GSK-3 tyrosine phosphorylation, but not basal tyrosine phosphorylation. Conversely, the Ca2+ ionophore ionomycin mimicked LPA in stimulating GSK-3 tyrosine phosphorylation (Figure 3D). From these results we conclude that tyrosine phosphorylation of GSK-3 is mediated by the PLC-Ca2+ mobilization pathway.
Figure 3.
GSK-3 tyrosine phosphorylation in B103-LPA1 cells mediated by Gi, PIP2 hydrolysis and Ca2+ mobilization. (A) FRET analysis of PIP2 hydrolysis in single B103-LPA1 cells treated with LPA (5 μM), thrombin receptor-activating peptide (TRP; 100 μM) and ionomycin (1 μM) in the absence or presence of PTX (200 ng/ml; overnight incubation). FRET between CFP-PH and YFP-PH is indicative of PH domain binding to PIP2 and is represented as the fluorescent YFP/CFP signal ratio (530 nm/475 nm; van der Wal et al., 2001). (B) Increase in cytosolic [Ca2+] induced by LPA (5 μM), TRP (100 μM), or ionomycin (1 μM) in the absence or presence of PTX (200 ng/ml; overnight). Peak value of the LPA-induced Ca2+ signal was defined as 100 “arbitrary units” (a.u.). (C) Effect of PTX pretreatment on GSK-3 tyrosine phosphorylation induced by either LPA or TRP. (D) Left, effect of BAPTA-AM (0.3 mM; 15 min) on LPA-induced GSK-3 tyrosine phosphorylation. Right: effect of ionomycin (1 μM, 1 min) on GSK-3 tyrosine phosphorylation. LPA (5 μM) was added for 2 min.
LPA-induced GSK-3 Tyrosine Phosphorylation Is Mediated by Pyk2
The proline-rich tyrosine kinase Pyk2 is a Ca2+-dependent tyrosine kinase related to focal adhesion kinase that is regulated by various extracellular signals (Lev et al., 1995; Dikic et al., 1996; Yu et al., 1996; Avraham et al., 2000; Park et al., 2000). Hence Pyk2 is a strong candidate for mediating tyrosine phosphorylation of GSK-3. Pyk2 is highly expressed in brain and neuronal cell lines (Menegon et al., 1999) and has been implicated in synaptic plasticity and neurite outgrowth (Ivankovic-Dikic et al., 2000; Huang et al., 2001). Activation of Pyk2 by GPCR agonists, including LPA, has previously been documented (Dikic et al., 1996; Andreev et al., 2001; Wu et al., 2002); moreover, it has been reported that Pyk2 can phosphorylate GSK-3 in vitro and in an CHO cell overexpression system (Hartigan et al., 2001).
Among various tyrosine kinase inhibitors, we found tyrphostin A9 to block the LPA-induced tyrosine phosphorylation of GSK-3 (but not basal tyrosine phosphorylation) in B103-LPA1 cells. Tyrphostin A9 is an inhibitor of Pyk2 signaling (Fuortes et al., 1999; Loeser et al., 2003), although it was originally identified as an inhibitor of the platelet-derived growth factor (PDGF) receptor tyrosine kinase (Levitzki and Gilon, 1991). Other tyrosine kinase inhibitors had no effect, including AG1296 (specific for the PDGF receptor), PP2 (selective for Src family members) and STI571 (Gleevec), an inhibitor of c-Abl and modulator of neurite morphology (Woodring et al., 2002; Figure 4A). The inhibitory effect of tyrphositin A9 was not unique for LPA as it was also observed with TRP and S1P (Figure 4B).
Figure 4.
Effect of protein tyrosine kinase inhibitors on LPA-induced GSK-3 tyrosine phosphorylation. (A) B103-LPA1 cells were treated without or with the indicated tyrosine kinase inhibitors for 15-30 min and subsequently treated with LPA (5 μM). Concentrations used: tyrphostin A9, 5 μM; AG1296, 2.5 μM; PP2, 1 μM; and STI571, 3 μM. (B) Effects of tyrphostin A9 (5 μM, 30 min) on GSK-3 tyrosine phosphorylation in response to TRP (100 μM) and S1P (200 nM).
Pyk2 residue Tyr-402 has been identified as the key site for autophosphorylation and kinase activation (Lev et al., 1995; Dikic et al., 1996). Using Tyr-402 phosphorylation as a readout, we found that LPA rapidly activates Pyk2 in B103-LPA1 cells (Figure 5A), which was confirmed by immunoprecipitation using anti-phosphotyrosine antibody followed by Western blotting using anti-Pyk2 antibody (Figure 5B). LPA-induced Pyk2 activity was blocked by PTX and BAPTA-AM (Figure 5, C and D), consistent with LPA1 mediating Pyk2 activation through a Gi-mediated rise in cytosolic Ca2+.
Figure 5.
Activation of Pyk2 by LPA in B103-LPA1 cells. (A) Autophosphorylation of Pyk2 at residue Y402 was analyzed using a phospho-specific antibody. An antibody against total Pyk2 was used for the loading control. LPA concentration, 5 μM. (B) Immunoprecipitation (IP) using anti-phosphotyrosine antibody 4G10 followed by immunoblotting (IB) using antibody against total Pyk2. Bottom blot shows Pyk2 expression levels in total cell lysates. (C) Immunoblot analysis of phospho-Pyk2(Y402) in cells pretreated or not with PTX (200 ng/ml overnight) and then treated with LPA for the indicated time periods. (D) Effect of BAPTA-AM (0.3 mM, 15 min) on basal and LPA-induced autophosphorylation of Pyk2.
If Pyk2 phosphorylates GSK-3, both kinases should physically interact. Pyk2 activation and GSK-3 tyrosine phosphorylation are rapid and transient, making it difficult to detect an endogenous association. However, when overexpressed in B103-LPA1 cells, Pyk2 could be coimmunoprecipitated with GSK-3β (Figure 6A). The reciprocal precipitation yielded inconsistent results, however. We therefore turned to Neuro2A cells, in which the presence of GSK-3β in Pyk2 precipitates (and vice versa) was more readily detectable (Figure 6B). The interaction between Pyk2 and GSK-3β did not require Pyk2 activity, as kinase-dead Pyk2(K457A) also interacted with GSK-3β (Figure 6A).
Figure 6.
Interaction between GSK-3β and Pyk2. (A) B103-LPA1 cells were transfected with GSK-3β-Myc alone or along with either Pyk2-GFP or with kinase-dead Pyk2-GFP (Pyk2KD-GFP). (B) Neuro-2A cells were transfected with either Pyk2-GFP alone or along with either Myc-tagged GSK-3β (GSK-3β-Myc) or GSK-3β-Myc together with GFP. Immunoprecipitation (IP; 48 h posttransfection) and immunoblotting (IB) were done using antibodies against GFP or Myc, as indicated.
To further substantiate the involvement of Pyk2 in LPA1-mediated GSK-3 tyrosine phosphorylation, we used “dominant-negative” Pyk2(K457A) and siRNAs against Pyk2. As shown in Figure 7A, transient introduction of Pyk2(K457A) into B103-LPA1 cells blocked GSK-3 tyrosine phosphorylation in response to LPA. Knockdown of Pyk2 expression was achieved by using the expression vector pSUPER (pS) that directs stable expression of siRNAs (Brummelkamp et al., 2002). Four different GFP-containing constructs were created to target rat Pyk2 mRNA (with GFP expressed from a distinct PGK promotor), termed pS-GFP-Pyk2. Targeting efficacy was tested in HEK293 cells by cotransfecting Pyk2-Myc and pS-GFP-Pyk2. Constructs pS-GFP-Pyk2-3 and pS-GFP-Pyk2-4 were found to knockdown Pyk2 expression by >90% (4 d after transfection; unpublished data). Because knockdown of endogenous Pyk2 expression was ∼50% in B103-LPA1 cells, these constructs were cotransfected with Myc-tagged Pyk2 to examine the effect on GSK-3 tyrosine phosphorylation. As shown in Figure 7B, both targeting constructs inhibited LPA1-mediated tyrosine phosphorylation of GSK-3 in B103-LPA1 cells.
Figure 7.

Inactivation or siRNA-mediated knockdown of Pyk2 inhibits LPA1-mediated GSK-3 tyrosine phosphorylation. (A) Top, B103-LPA1 cells transfected with either GFP (control) or GFP-tagged kinase-dead Pyk2 (Pyk2KD-GFP) were treated with LPA (5 μM, 5 min) and tyrosine-phosphorylated GSK-3 was analyzed by immunoblotting. Bottom, densitometric data from six experiments represented as the ratio between PY-GSK3α/β and total GSK-3 α/β. Error bars, SD. (B) Top, B103-LPA1 cells were transfected with Pyk2-Myc together with either pS-GFP or the siRNA-encoding constructs pS-GFP-Pyk2-3 or pS-GFP-Pyk2-4, as indicated. At 72 h after transfection, cells were treated with LPA (5 μM, 5 min) and both Pyk2-Myc expression and GSK-3 tyrosine phosphorylation were analyzed by immunoblotting. Bottom, ratio between densitometric values of PY-GSK3α/β and total GSK-3α/β from six experiments.
To confirm the Pyk2-GSK-3 connection in other neuronal cells, we screened various cell lines for Pyk2 expression. SH-SY5Y cells were found to lack detectable Pyk2, in agreement with previous findings (Ivankovic-Dikic et al., 2000), whereas PC12 cells show high expression levels (Figure 8A). In the latter cells, LPA induces GSK-3 tyrosine phosphorylation concurrent with Pyk2 activation (Figure 8B), although the response is relatively weak. Knockdown of endogenous Pyk2, using an adenoviral construct expressing RNAi3, abolished LPA-induced GSK-3 tyrosine phosphorylation in PC12 cells (and partially inhibited basal GSK-3 tyrosine phosphorylation; Figure 8C). In Pyk2-deficient SH-SY5Y cells, LPA failed to increase GSK-3 tyrosine phosphorylation above basal levels (Figure 8D); but after forced expression of Pyk2, LPA-induced GSK-3 tyrosine phosphorylation was readily detectable (Figure 8E). Thus, Pyk2 mediates LPA-induced tyrosine phosphorylation of GSK-3 in different neuronal cell types.
Figure 8.
Involvement of Pyk2 in LPA-induced GSK-3 phosphorylation in PC12 and SH-SY5Y cells. (A) Pyk2 expression in PC12, SH-SY5Y, and B103-LPA1 cells. (B) PC12 cells were treated with LPA (5 μM) for different time periods. Expression and phosphotyrosine levels of Pyk2 and GSK-3α/β were determined by immunoblotting. (C) GSK-3 phosphotyrosine in PC12 cells infected with either control (empty) or RNAi3 expressing adenoviruses and treated with LPA (5 μM) for 5 min. (D) SH-SY5Y neuroblastoma cells (differentiated with db-cAMP) were treated with LPA (5 μM) for the indicated time periods. GSK-3α/β phosphotyrosine and total GSK-3 levels were analyzed by immunoblotting. (E) SH-SY5Y cells stably overexpressing Pyk2-GFP or GFP alone were treated with LPA (5 μM) for the indicated time periods and phosphotyrosine levels in GSK-3α/β were analyzed by immunoblotting. Total GSK-3α/β was used as a loading control.
Inhibition of Pyk2 and GSK-3 Interferes with LPA1-mediated Neurite Retraction
Because GSK-3 activation by LPA coincides with neurite retraction, we examined whether the Pyk2-GSK-3 pathway is involved in neurite remodeling in B103-LPA1 cells. Three different pharmacological inhibitors of GSK-3 (lithium, SB-415286 and SB-216763) suppressed LPA1-mediated neurite retraction and cell rounding (Figure 9, A and B). The number of contracted cells (at 15 min after LPA addition) dropped from 65% in untreated cells to ∼35% in cells treated with the GSK-3 inhibitors (Figure 9A). In addition, expression of “dominant-negative” (kinase-inactive [KI]) GSK-3β also interfered with LPA-induced contraction (Figure 9C). Thus, GSK-3 activity is required for optimal neurite retraction.
Similar to what is observed with the GSK-3 inhibitors, the Pyk2 inhibitor tyrphostin A9 partially inhibited LPA-induced neurite retraction (Figure 10A, left panel). About 90% of the control cells underwent neurite retraction within 30 min of LPA addition, whereas only 47% of the A9-pretreated cells showed a contractile response (Figure 10A, right panel). Knockdown of Pyk2 mRNA in B103-LPA1 cells, using construct pS-GFP-Pyk2-4, induced the formation of neuritelike extensions that were partially resistant to LPA (Figure 10B). Together, these findings indicate that the Pyk2-GSK-3 pathway is required for optimal neurite retraction in response to LPA.
Figure 10.
Involvement of Pyk2 in LPA1-induced neurite retraction. (A) B103-LPA1 cells were pretreated or not with tyrphostin A9 (5 μM) for 30 min before LPA addition (5 μM, 30 min). Pictures were taken of the same field of cells before and after LPA stimulation. Right, quantification of the percentage of contraction after LPA exposure in control cells and cells pretreated with tyrphostin A9. Data represent the means (±SD) of four different experiments. (B) B103-LPA1 cells were transfected with RNAi construct pS-GFP-Pyk2-4 or pS-GFP. Four days after transfection cells were stimulated with LPA (5 μM). Fluorescence pictures of GFP-positive cells were taken of the same field before and after LPA addition (30 min). Right, quantification of the percentage of contraction after LPA exposure in cells transfected with GFP or with Pyk2 targeting RNAi construct pS-GFP-Pyk2-4. Data represent the means (±SD) of six different experiments.
DISCUSSION
GSK-3 is commonly considered as a constitutively active enzyme that is regulated through inhibitory serine phosphorylation. Increasing evidence suggests, however, that GSK-3 activity is increased by tyrosine phosphorylation. In particular, previous studies have shown that GSK-3 activity is increased after stimulation of neuronal cells with LPA (Sayas et al., 1999, 2002b), a potent inducer of Gα12/13-RhoA-mediated growth cone collapse and neurite retraction. However, the signaling events and the tyrosine kinase(s) responsible for GSK-3 activation by LPA have remained unclear.
Growth cone collapse and neurite retraction in response to repulsive cues are essential to achieve correct neural connectivity during nervous system development; furthermore, neurite retraction occurs during neurodegeneration and could also be important during traumatic brain injury, when neurons are suddenly exposed to blood-borne factors such as LPA. In the present study, we have examined how LPA1, the predominant LPA receptor in the nervous system, induces stimulatory tyrosine phosphorylation of GSK-3, a response leading to phosphorylation of tau and accompanying neurite retraction. LPA1 signaling has been implicated in neurogenesis and suppression of apoptosis during brain development (Fukushima et al., 2001; Kingsbury et al., 2003), while it leads to neurite retraction and cell migration in cultured neuronal cells (Van Leeuwen et al., 2003). We show here that LPA1 stimulates tyrosine phosphorylation of GSK-3 through the Ca2+-sensitive tyrosine kinase Pyk2 downstream of the Gi-linked PLC-Ca2+ mobilization pathway. Our conclusion is based on the following findings: 1) LPA-induced tyrosine phosphorylation of GSK-3 is inhibited by chelation of intracellular Ca2+ and mimicked by the Ca2+ ionophore ionomycin; 2) LPA activates Pyk2 in a pertussis toxin- and Ca2+-dependent manner and with kinetics similar to those of GSK-3; moreover, Pyk2 and GSK-3β exist in a complex as both enzymes can be coimmunoprecipitated from cell lysates; 3) pharmacological inhibition, kinase-dead Pyk2 as well as siRNAs against Pyk2 interfere with LPA1-mediated tyrosine phosphorylation of GSK-3; and 4) LPA fails to increase GSK-3 tyrosine phosphorylation in Pyk2-deficient SH-SY5Y neuroblastoma cells, whereas the response is restored after forced expression of Pyk2; yet LPA does activate GSK-3 in SH-SY5Y cells (Sayas et al., 1999), suggesting the existence of an alternative mechanism of GSK-3 activation, presumably serine dephosphorylation as is observed with the axonal repellent Sema3A (Eickholt et al., 2002). Other collapsing factors, notably thrombin and S1P, provoke the same signaling events as LPA, suggesting that the Pyk2-GSK-3 pathway is a general component of the neurite retraction response. Thus, different repulsive/collapsing agonists converge at GSK-3 to increase its activity through tyrosine phosphorylation or/and serine dephosphorylation.
We find that pharmacological inhibition or knockdown of Pyk2 partially inhibits LPA1-mediated neurite retraction in B103-LPA1 cells, as is also observed with three structurally unrelated inhibitors of GSK-3 and a dominant-negative (kinase-dead) version of GSK-3β, suggesting that the Pyk2-GSK-3 pathway is necessary for full neurite retraction. Previous studies on PC12 cells have implicated Pyk2 in the regulation of neurite outgrowth following costimulation of growth factor receptors and integrins (Ivankovic-Dikic et al., 2000; Haglund et al., 2004), whereas another study has suggested involvement of Pyk2 in neurite retraction (Park et al., 2000). Supporting evidence for a role for Pyk2 in cytoskeletal contraction comes from Pyk2 knockout studies showing that Pyk2 is essential for lamellipodial contractile activity in macrophages (Okigaki et al., 2003). It thus seems that the activity of Pyk2 may increase during retraction as well as outgrowth of developing neurites, likely depending on its subcellular localization and specific binding partners.
Precisely how Pyk2-activated GSK-3 participates in neurite retraction remains to be clarified, but it seems likely that GSK-3 primarily acts by promoting microtubule dynamics through its ability to phosphorylate microtubule-binding proteins implicated in axon growth, notably tau, CRMP-2, APC, and MAP1B (Zhou and Snider, 2005). Microtubules constitute the main cytoskeletal component in the shaft of developing neurites and should disassemble during neurite retraction. We propose that the Pyk2-GSK-3-microtubule destabilization pathway functions in parallel and cooperates with the force-generating RhoA-ROCK-actomyosin pathway to induce optimal neurite retraction, as schematically illustrated in Figure 11.
Figure 11.
Schematic model of GSK-3 activation by LPA1. After stimulation of LPA1 or the GPCRs for S1P and thrombin, GSK-3 is activated by the tyrosine kinase Pyk2 downstream of Gi/Gq-mediated PLC activation and Ca2+ mobilization. Parallel to the force-generating Gα12/13-RhoA-ROCK-actomyosin pathway, GSK-3 phosphorylates microtubule-associated proteins, such as tau, MAP1b, CRMP-2, and APC, on Ser/Thr to enhance microtubule dynamics. We note that GSK-3 can also be activated through Gα12/13 (Sayas et al., 2002b). PLC, phospholipase C; ROCK, Rho-kinase; MLC, myosin light chain.
Structural and biochemical studies have suggested that tyrosine phosphorylation of GSK-3 may serve to facilitate substrate recognition or/and to enhance the stability of the enzyme (Dajani et al., 2001; Cole et al., 2004). It has been argued that basal tyrosine phosphorylation of GSK-3 represents an intramolecular autophosphorylation event (Cole et al., 2004) but, as we show here, agonist-induced tyrosine phosphorylation is not. Pharmacological inhibition of GSK-3, using either LiCl or SB-216763, did not suppress LPA-induced GSK-3 tyrosine phosphorylation (unpublished data), indicating that basal kinase activity is not required for GSK-3 to become tyrosine phosphorylated by LPA. Furthermore, we find that tyrosine kinase inhibitors interfere with agonist-induced GSK-3 tyrosine phosphorylation, but not with basal tyrosine phosphorylation. In other words, agonist-induced tyrosine phosphorylation of GSK-3 can be dissociated from its basal tyrosine phosphorylation. (Yet in PC12 cells, Pyk2 knockdown did reduce basal GSK-3 phosphotyrosine levels). Whether basal tyrosine phosphorylation of GSK-3 represents primarily an autocatalytic event or is mediated by another kinase remains to be established.
In a previous study, using activated Gα subunits, it was reported that GSK-3 activation in neuronal cells occurs downstream of Gα12/13 through Rho-dependent and -independent pathways (Sayas et al., 2002a); however, possible involvement of the PLC-Ca2+ mobilization pathway was not examined. Yet it remains possible that LPA can bypass the PLC pathway to activate Pyk2/GSK-3 via enhanced Ca2+ influx (as opposed to Ca2+ mobilization from internal stores). For example, LPA evokes a prominent Gα13-mediated membrane depolarization (Postma et al., 2001), which in turn could stimulate voltage-dependent Ca2+ entry and subsequent activation of Pyk2/GSK-3.
We find that LPA phosphorylates GSK-3(α/β) not only on tyrosine but also on serine (Ser-21 and Ser-9, respectively). But compared with the strong and sustained phosphorylation induced by insulin, LPA is relatively weak in stimulating GSK-3 serine phosphorylation in B103-LPA1 cells. Probably only a small proportion of GSK-3 molecules is serine phosphorylated by LPA. Thus, the net result of LPA1-mediated dual tyrosine/serine phosphorylation of GSK-3 is an increase in catalytic activity above basal levels. Interestingly, treatment of primary neurons with a neurotoxic prion peptide also induces dual serine/tyrosine phosphorylation of GSK-3 with a net increase in activity (Perez et al., 2003). By contrast, in Swiss 3T3 cells LPA is a relatively strong inducer of GSK-3 serine phosphorylation leading to inactivation of the enzyme (Fang et al., 2002). Reconstitution experiments in HEK293 cells suggest that GSK-3 serine phosphorylation is mediated by LPA2 and LPA3, but not LPA1 (Fang et al., 2002). Although the authors did not analyze the tyrosine phosphorylation state of GSK-3, the discrepancy with the present results is most likely due to differences in cell type (neuronal vs. nonneuronal), LPA receptor expression profile and the relative strength of the corresponding intracellular signals.
In conclusion, we have defined a PLC-Ca2+-Pyk2 pathway that couples LPA1 to GSK-3 tyrosine phosphorylation and activation in neuronal cells. As one would predict, other collapsing (i.e., RhoA-activating) and PIP2-hydrolyzing GPCR agonists, notably thrombin and S1P, also induce GSK-3 tyrosine phosphorylation concomitant with neurite retraction. It thus appears that transient tyrosine phosphorylation and activation of GSK-3, with subsequent phosphorylation of microtubule-associated proteins, is one of the initial steps during neurite retraction induced by GPCRs that signal via both RhoA and PLC. Both Pyk2 and GSK-3 are highly expressed in the developing nervous system as well as adult brain, where they are thought to play important roles in synapse formation and plasticity (Henley and Nishimune, 2001; Tokuoka et al., 2002). In cultured neuronal cells, GSK-3 and Pyk2 appear to be enriched in neurites and growth cones (Menegon et al., 1999; Zhou et al., 2004). Pyk2 may regulate neuronal GSK-3 activity in vivo, because ischemic brain injury leads to Pyk2 activation (Tian et al., 2000) as well as GSK-3 tyrosine phosphorylation (Bhat et al., 2000), consistent with Pyk2 phosphorylating GSK-3 under pathological conditions. It will be of interest to examine whether Pyk2 activity in brain is deregulated in Alzheimer patients or after brain injury and thereby may lead to undue activation of GSK-3.
Acknowledgments
We thank Paula Ruurs and Trudi Hengeveld for technical assistance; Novartis for providing STI571 (Gleevec); Drs. Sancho, Sanchez Madrid, Schlessinger, Woodgett, and Earp for providing expression vectors; Drs. Avila and Davies for antibodies; and Drs. Wandosell and Moreno-Flores for useful comments. This work was supported by an EC Marie Curie fellowship FP5 to C.L.S. (HPMF-CT-2002-01988) and the Dutch Cancer Society.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-07-0688) on February 1, 2006.
Abbreviations used: GSK-3, glycogen synthase kinase-3; LPA, lysophosphatidic acid; Pyk2, proline-rich tyrosine kinase 2.
References
- Andreev, J., Galisteo, M. L., Kranenburg, O., Logan, S. K., Chiu, E. S., Okigaki, M., Cary, L. A., Moolenaar, W. H., and Schlessinger, J. (2001). Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J. Biol. Chem. 276, 20130-20135. [DOI] [PubMed] [Google Scholar]
- Avraham, H., Park, S. Y., Schinkmann, K., and Avraham, S. (2000). RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 12, 123-133. [DOI] [PubMed] [Google Scholar]
- Bhat, R. V., Shanley, J., Correll, M. P., Fieles, W. E., Keith, R. A., Scott, C. W., and Lee, C. M. (2000). Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA 97, 11074-11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp, T. R., Bernards, R., and Agami, R. (2002). Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2, 243-247. [DOI] [PubMed] [Google Scholar]
- Chun, J., Goetzl, E. J., Hla, T., Igarashi, Y., Lynch, K. R., Moolenaar, W., Pyne, S., and Tigyi, G. (2002). International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 54, 265-269. [DOI] [PubMed] [Google Scholar]
- Cohen, P. and Goedert, M. (2004). GSK3 inhibitors: development and therapeutic potential. Nat. Rev. Drug Discov. 3, 479-487. [DOI] [PubMed] [Google Scholar]
- Cole, A., Frame, S., and Cohen, P. (2004). Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 377, 249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, D. A., Alessi, D. R., Cohen, P., Andjelkovich, M., and Hemmings, B. A. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785-789. [DOI] [PubMed] [Google Scholar]
- Dajani, R., Fraser, E., Roe, S. M., Young, N., Good, V., Dale, T. C., and Pearl, L. H. (2001). Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105, 721-732. [DOI] [PubMed] [Google Scholar]
- Dikic, I., Tokiwa, G., Lev, S., Courtneidge, S. A., and Schlessinger, J. (1996). A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383, 547-550. [DOI] [PubMed] [Google Scholar]
- Doble, B. W. and Woodgett, J. R. (2003). GSK-3, tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes, G. (2004). MARKing tau for tangles and toxicity. Trends Biochem. Sci. 29, 548-555. [DOI] [PubMed] [Google Scholar]
- Eickholt, B. J., Walsh, F. S., and Doherty, P. (2002). An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J. Cell Biol. 157, 211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, G., Ballou, L. M., and Lin, R. Z. (2003). Phospholipase C-independent activation of glycogen synthase kinase-3beta and C-terminal Src kinase by Galphaq. J. Biol. Chem. 278, 52432-52436. [DOI] [PubMed] [Google Scholar]
- Fang, X., Yu, S., Tanyi, J. L., Lu, Y., Woodgett, J. R., and Mills, G. B. (2002). Convergence of multiple signaling cascades at glycogen synthase kinase 3, Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a PKC-dependent intracellular pathway. Mol. Cell. Biol. 22, 2099-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, X., Yu, S. X., Lu, Y., Bast, R. C., Jr., Woodgett, J. R., and Mills, G. B. (2000). Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. USA 97, 11960-11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame, S. and Cohen, P. (2001). GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima, N., Ishii, I., Contos, J. J., Weiner, J. A., and Chun, J. (2001). Lysophospholipid receptors. Annu. Rev. Pharmacol. Toxicol. 41, 507-534. [DOI] [PubMed] [Google Scholar]
- Fuortes, M., Melchior, M., Han, H., Lyon, G. J., and Nathan, C. (1999). Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J. Clin. Invest 104, 327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes, C. A. and Jope, R. S. (2001). The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 65, 391-426. [DOI] [PubMed] [Google Scholar]
- Haglund, K., Ivankovic-Dikic, I., Shimokawa, N., Kruh, G. D., and Dikic, I. (2004). Recruitment of Pyk2 and Cbl to lipid rafts mediates signals important for actin reorganization in growing neurites. J. Cell Sci. 117, 2557-2568. [DOI] [PubMed] [Google Scholar]
- Hanger, D. P., Hughes, K., Woodgett, J. R., Brion, J. P., and Anderton, B. H. (1992). Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci. Lett. 147, 58-62. [DOI] [PubMed] [Google Scholar]
- Hartigan, J. A. and Johnson, G. V. (1999). Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J. Biol. Chem. 274, 21395-21401. [DOI] [PubMed] [Google Scholar]
- Hartigan, J. A., Xiong, W. C., and Johnson, G. V. (2001). Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem. Biophys. Res. Commun. 284, 485-489. [DOI] [PubMed] [Google Scholar]
- Henley, J. M. and Nishimune, A. (2001). CAKbeta/Pyk2 activates Src: another piece in the puzzle of LTP induction. Neuron 29, 312-314. [DOI] [PubMed] [Google Scholar]
- Hirose, M., Ishizaki, T., Watanabe, N., Uehata, M., Kranenburg, O., Moolenaar, W. H., Matsumura, F., Maekawa, M., Bito, H., and Narumiya, S. (1998). Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J. Cell Biol. 141, 1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. et al. (2001). CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron 29, 485-496. [DOI] [PubMed] [Google Scholar]
- Hughes, K., Nikolakaki, E., Plyte, S. E., Totty, N. F., and Woodgett, J. R. (1993). Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 12, 803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic-Dikic, I., Gronroos, E., Blaukat, A., Barth, B. U., and Dikic, I. (2000). Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat. Cell Biol. 2, 574-581. [DOI] [PubMed] [Google Scholar]
- Jalink, K., Hengeveld, T., Mulder, S., Postma, F. R., Simon, M.F., Chap, H., van der Marel, G. A., van Boom, J. H., van Blitterswijk, W. J., and Moolenaar, W. H. (1995). Lysophosphatidic acid-induced Ca2+ mobilization in human A431 cells: structure-activity analysis. Biochem. J. 307(Pt 2), 609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink, K., van Corven, E. J., Hengeveld, T., Morii, N., Narumiya, S., and Moolenaar, W. H. (1994). Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein. Rho. J. Cell Biol. 126, 801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink, K., van Corven, E. J., and Moolenaar, W. H. (1990). Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J. Biol. Chem. 265, 12232-12239. [PubMed] [Google Scholar]
- Jiang, H., Guo, W., Liang, X., and Rao, Y. (2005). Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell 120, 123-135. [DOI] [PubMed] [Google Scholar]
- Jope, R. S. and Johnson, G. V. (2004). The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29, 95-102. [DOI] [PubMed] [Google Scholar]
- Kim, L., Liu, J., and Kimmel, A. R. (1999). The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell 99, 399-408. [DOI] [PubMed] [Google Scholar]
- Kingsbury, M. A., Rehen, S. K., Contos, J. J., Higgins, C. M., and Chun, J. (2003). Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat. Neurosci. 6, 1292-1299. [DOI] [PubMed] [Google Scholar]
- Kranenburg, O., Poland, M., van Horck, F. P., Drechsel, D., Hall, A., and Moolenaar, W. H. (1999). Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol. Biol. Cell 10, 1851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, K. and Brion, J. P. (1999). Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J. Chem. Neuroanat. 16, 279-293. [DOI] [PubMed] [Google Scholar]
- Lesort, M., Jope, R. S., and Johnson, G. V. (1999). Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J. Neurochem. 72, 576-584. [DOI] [PubMed] [Google Scholar]
- Lev, S., Moreno, H., Martinez, R., Canoll, P., Peles, E., Musacchio, J. M., Plowman, G. D., Rudy, B., and Schlessinger, J. (1995). Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 376, 737-745. [DOI] [PubMed] [Google Scholar]
- Levitzki, A. and Gilon, C. (1991). Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol. Sci. 12, 171-174. [DOI] [PubMed] [Google Scholar]
- Loeser, R. F., Forsyth, C. B., Samarel, A. M., and Im, H. J. (2003). Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a PKC-dependent pathway. J. Biol. Chem. 278, 24577-24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon, A., Burgaya, F., Baudot, P., Dunlap, D. D., Girault, J. A., and Valtorta, F. (1999). FAK+ and PYK2/CAKbeta, two related tyrosine kinases highly expressed in the central nervous system: similarities and differences in the expression pattern. Eur. J. Neurosci. 11, 3777-3788. [DOI] [PubMed] [Google Scholar]
- Moolenaar, W. H., van Meeteren, L. A., and Giepmans, B. N. (2004). The ins and outs of lysophosphatidic acid signaling. Bioessays 26, 870-881. [DOI] [PubMed] [Google Scholar]
- Mukai, F., Ishiguro, K., Sano, Y., and Fujita, S. C. (2002). Alternative splicing isoform of tau protein kinase I/glycogen synthase kinase 3beta. J. Neurochem. 81, 1073-1083. [DOI] [PubMed] [Google Scholar]
- Mulder, J., Ariaens, A., van den Boomen, D., and Moolenaar, W. H. (2004). p116Rip targets myosin phosphatase to the actin cytoskeleton and is essential for RhoA/ROCK-regulated neuritogenesis. Mol. Biol. Cell 15, 5516-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, K., Ishii, S., and Shimizu, T. (2003). Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 278, 25600-25606. [DOI] [PubMed] [Google Scholar]
- Okigaki, M., Davis, C., Falasca, M., Harroch, S., Felsenfeld, D. P., Sheetz, M. P., and Schlessinger, J. (2003). Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. USA 100, 10740-10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. Y., Avraham, H., and Avraham, S. (2000). Characterization of the tyrosine kinases RAFTK/Pyk2 and FAK in nerve growth factor-induced neuronal differentiation. J. Biol. Chem. 275, 19768-19777. [DOI] [PubMed] [Google Scholar]
- Perez, M., Rojo, A. I., Wandosell, F., Diaz-Nido, J., and Avila, J. (2003). Prion peptide induces neuronal cell death through a pathway involving glycogen synthase kinase. Biochem. J. 372, 129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, F. R., Jalink, K., Hengeveld, T., Offermanns, S., and Moolenaar, W. H. (2001). Galpha(13) mediates activation of a depolarizing chloride current that accompanies RhoA activation in both neuronal and nonneuronal cells. Curr. Biol. 11, 121-124. [DOI] [PubMed] [Google Scholar]
- Sayas, C. L., Avila, J., and Wandosell, F. (2002a). Regulation of neuronal cytoskeleton by lysophosphatidic acid: role of GSK-3. Biochim. Biophys. Acta 1582, 144-153. [DOI] [PubMed] [Google Scholar]
- Sayas, C. L., Avila, J., and Wandosell, F. (2002b). Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms. J. Neurosci. 22, 6863-6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayas, C. L., Moreno-Flores, M. T., Avila, J., and Wandosell, F. (1999). The neurite retraction induced by lysophosphatidic acid increases Alzheimer's disease-like Tau phosphorylation. J. Biol. Chem. 274, 37046-37052. [DOI] [PubMed] [Google Scholar]
- Schaffer, B., Wiedau-Pazos, M., and Geschwind, D. H. (2003). Gene structure and alternative splicing of glycogen synthase kinase 3 beta (GSK-3beta) in neural and non-neural tissues. Gene 302, 73-81. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Tomizawa, K., Kato, R., Sato, K., Uchida, T., Fujita, S. C., and Imahori, K. (1994). Localization and developmental changes of tau protein kinase I/glycogen synthase kinase-3 beta in rat brain. J. Neurochem. 63, 245-255. [DOI] [PubMed] [Google Scholar]
- Tian, D., Litvak, V., and Lev, S. (2000). Cerebral ischemia and seizures induce tyrosine phosphorylation of PYK2 in neurons and microglial cells. J. Neurosci. 20, 6478-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuoka, H., Yoshida, T., Matsuda, N., and Mishina, M. (2002). Regulation by glycogen synthase kinase-3beta of the arborization field and maturation of retinotectal projection in zebrafish. J. Neurosci. 22, 10324-10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi, N., Marsh, P., Goold, R. G., Wood-Kaczmar, A., and Gordon-Weeks, P. R. (2005). Glycogen synthase kinase-3beta phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. J. Cell Sci. 118, 993-1005. [DOI] [PubMed] [Google Scholar]
- van der Wal, J., Habets, R., Varnai, P., Balla, T., and Jalink, K. (2001). Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J. Biol. Chem. 276, 15337-15344. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen, F. N., Olivo, C., Grivell, S., Giepmans, B. N., Collard, J. G., and Moolenaar, W. H. (2003). Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. J. Biol. Chem. 278, 400-406. [DOI] [PubMed] [Google Scholar]
- Wagner, U., Utton, M., Gallo, J. M., and Miller, C. C. (1996). Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J. Cell Sci. 109(Pt 6), 1537-1543. [DOI] [PubMed] [Google Scholar]
- Wang, Q. M., Fiol, C. J., DePaoli-Roach, A. A., and Roach, P. J. (1994). Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J. Biol. Chem. 269, 14566-14574. [PubMed] [Google Scholar]
- Welsh, G. I., Patel, J. C., and Proud, C. G. (1997). Peptide substrates suitable for assaying glycogen synthase kinase-3 in crude cell extracts. Anal. Biochem. 244, 16-21. [DOI] [PubMed] [Google Scholar]
- Woodgett, J. R. (1990). Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 9, 2431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodring, P. J., Litwack, E. D., O'Leary, D. D., Lucero, G. R., Wang, J. Y., and Hunter, T. (2002). Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J. Cell Biol. 156, 879-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. S., Chiu, T., and Rozengurt, E. (2002). ANG II and LPA induce Pyk2 tyrosine phosphorylation in intestinal epithelial cells: role of Ca2+, PKC, and Rho kinase. Am. J. Physiol. Cell Physiol. 282, C1432-C1444. [DOI] [PubMed] [Google Scholar]
- Yoshimura, T., Kawano, Y., Arimura, N., Kawabata, S., Kikuchi, A., and Kaibuchi, K. (2005). GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120, 137-149. [DOI] [PubMed] [Google Scholar]
- Yu, H. et al. (1996). Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J. Biol. Chem. 271, 29993-29998. [DOI] [PubMed] [Google Scholar]
- Zhou, F. Q. and Snider, W. D. (2005). Cell biology. GSK-3beta and microtubule assembly in axons. Science 308, 211-214. [DOI] [PubMed] [Google Scholar]
- Zhou, F. Q., Zhou, J., Dedhar, S., Wu, Y. H., and Snider, W. D. (2004). NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42, 897-912. [DOI] [PubMed] [Google Scholar]