Abstract
Transforming growth factor beta 1 (TGF-β1) has been shown to induce epithelial-mesenchymal transition (EMT) during various stages of embryogenesis and progressive disease. This alteration in cellular morphology is typically characterized by changes in cell polarity and loss of adhesion proteins such as E-cadherin. Here we demonstrate that EMT is associated with loss of claudin-1, claudin-2, occludin, and E-cadherin expression within 72 h of exposure to TGF-β1 in MDCKII cells. It has been suggested that this expression loss occurs through TGF-β1 in a Smad-independent mechanism, involving MEK and PI3K pathways, which have previously been shown to induce expression of the Snail (SNAI-1) gene. Here we show that these pathways are responsible for loss of tight junctions and a partial loss of E-cadherin. However, our results also demonstrate that a complete loss of E-cadherin and transformation to the mesenchymal phenotype are dependent on Smad signaling, which subsequently stimulates formation of β-catenin/LEF-1 complexes that induce EMT.
INTRODUCTION
Epithelial-mesenchymal transition (EMT) is a fundamental mechanism of development (Hay, 1995) that is responsible for initiating pathological conditions in the adult organism, such as tumor metastasis (Thiery, 2002) and organ fibrosis (Zeisberg and Kalluri, 2004). This cellular transformation results in loss of cell adhesion and apical-basal polarity, followed by a shift in cytoskeletal dynamics toward the mesenchymal phenotype. These newly formed mesenchymal cells will then migrate to specific destinations based on their developmental programming in the embryo or toward lymphatics and blood vessels during tumor metastasis (Nawshad et al., 2005).
EMT is most commonly assessed biochemically by loss of E-cadherin expression. Before the shift in cell polarity, cell-cell adhesion must be lost to produce individual migrating mesenchymal cells. Suppression of E-cadherin has been linked to various transcription factors such as Snail, Slug, SIP-1, ZEB-1, E12/E47 (Peinado et al., 2004), and Twist (Yang et al., 2004), which bind the promoter of the E-cadherin gene to repress its transcription. Snail has also been linked to loss of other junction proteins such as claudins, occludin, and ZO-1 (Ikenouchi et al., 2003; Ohkubo and Ozawa, 2004).
Another molecule that has recently been shown to suppress E-cadherin gene transcription is Lymphoid Enhancer Factor-1 (LEF-1; Jamora et al., 2003), a TCF (T-cell factor) transcription factor typically associated with Wnt/β-catenin signaling. β-Catenin/LEF-1 complexes play a key role in EMT as previously demonstrated in colon carcinomas (Kim et al., 2002). The Wnt signaling pathway has been established as a necessary component to drive EMT in several embryonic systems, such as neural crest formation (Garcia-Castro and Bronner-Fraser, 1999) and somitogenesis (Galceran et al., 2004). Wnt signaling promotes stabilization of cytoplasmic β-catenin through phosphorylation and functional deactivation of GSK-3β. β-Catenin is typically degraded through the ubiquitin proteosome pathway, which occurs via a complex of proteins including APC, Axin, and GSK-3β. Dissociation of this complex by phosphorylation of GSK-3β (through the Wnt signaling protein Dishevelled) increases pools of cytoplasmic β-catenin, which can then bind with LEF-1 to promote EMT (Kim et al., 2002; Waterman, 2004). Conveniently, GSK-3β phosphorylation has also been linked to proper function of the E-cadherin repressor protein Snail, which is degraded (much like β-catenin) by the ubiquitin complex (Zhou et al., 2004). Other signaling pathways linked to phosphoinositide-3-kinase (PI3K) can also induce phosphorylation of GSK-3β through such downstream molecules as ILK and AKT (Hannigan et al., 2005).
In the absence of Wnt, members of the transforming growth factor beta (TGF-β) family can promote EMT through cross-talk mechanisms with Wnt signaling molecules (Labbe et al., 2000; Nishita et al., 2000). TGF-β has been reported to induce the invasive phenotype in many epithelial cancers and is essential for promoting EMT at various stages of embryogenesis, such as neural crest formation and palatogenesis (Nawshad and Hay, 2003; Nawshad et al., 2005). Upon ligand-receptor binding of TGF-β to TβRII, heterodimerization occurs with TβRI (ALK5). Formation of this receptor complex is followed by endocytosis, a process mediated by Rab5. In the early endosome TβRI is susceptible to binding with the Smad Anchor for Receptor Activation (SARA), which recruits specific R-Smad proteins (Smad2 or 3). On phosphorylation by TβRI, the R-Smad is released from the receptor complex and is followed by association with Smad4. This Smad2/3-Smad4 complex then enters the nucleus to promote transcription of target genes (Shi and Massague, 2003; ten Dijke and Hill, 2004). Although Smads are powerful tumor suppressors because of their ability to prevent progression through G1 phase of the cell cycle via up-regulation of cyclin-dependent kinase inhibitors (Massague, 2004), they may also promote EMT (Akhurst and Derynck, 2001). Complexes of phosphorylated Smad2 and Smad4 have been shown to up-regulate expression of the LEF-1 transcription factor (Nawshad and Hay, 2003). LEF-1 can then associate with β-catenin or reassociate with Smads (Labbe et al., 2000), to transcribe EMT target genes (i.e., E-cadherin, Vimentin, Fibronectin, etc.; Nawshad and Hay, 2003; Nawshad et al., unpublished results).
TGF-β also has the ability to signal in a Smad-independent manner following pathways most commonly associated with receptor tyrosine kinases. Recent studies have shown that TβRI (ALK5) can directly bind and activate PI3K (Yi et al., 2005). Although the mechanism is not yet understood, the available data suggest that TGF-β can signal through Ras GTPase (Janda et al., 2002), a pathway that has been deemed as necessary (but not sufficient) for EMT in several systems (Xie et al., 2004). Also, TGF-β receptors can signal through Par6-Smurf1 to mediate ubiquitination of RhoA, an inhibitor of TGF-β-dependent EMT (Ozdamar et al., 2005).
In MDCKII cells, TGF-β1 has been shown to promote EMT via the Smad-independent Ras-Raf-MEK-ERK-AP-1 signaling pathway, which up-regulates transcription of the E-cadherin repressor gene, Snail. Subsequent suppression of E-cadherin correlates with up-regulation of mesenchymal markers (i.e., Vimentin and Fibronectin) and a definitive change in cellular morphology (Peinado et al., 2003). However, these findings in no way assessed the potential of Smad signaling in this transition. Our objective was to establish any possible role of Smad or β-catenin/LEF-1 pathways being involved in promoting EMT in cooperation with the previously described Smad-independent/Snail signaling.
MATERIALS AND METHODS
Cell Culture
The MDCKII cell line was acquired from American Tissue Culture Collection (ATCC, Rockville, MD) and grown in medium containing DMEM (Invitrogen) + 10% fetal bovine serum + 1% penicillin/streptomycin. Fetal bovine serum was removed for all experimental conditions. Recombinant TGF-β1 (R&D Systems, Minneapolis, MN) was added to the culture medium at a concentration of 10 ng/ml for all experiments. DN Smad4 (kindly provided by Dr. Diane Simeone, University of Michigan, Ann Arbor) and DN LEF-1 (kindly provided by Dr. Marian Waterman, University of California, Irvine) adenoviral constructs were added at concentrations of 1:100 for 24 h before treatment with TGF-β1. MEK1/2 inhibitor U0126 (at a concentration of 1:20) and PI3K inhibitor LY294002 (at a concentration of 1:50) (Invitrogen, Carlsbad, CA) were added for 1 h before treatment with TGF-β1.
Immunocytochemistry and Immunoblotting
Immunocytochemistry and Western blotting were performed as previously described by Li et al. (2002). The following antibodies were used at concentrations recommended by the respective manufacturer: p-Smad2/3, Smad4, LEF-1, Snail (SNAI-1; Santa Cruz Biotechnology, Santa Cruz, CA), claudin-1, claudin-2, occludin, E-cadherin (Zymed, South San Francisco, CA), β-catenin, Vimentin (Sigma, St. Louis, MO), p-ERK1/2, p-AKT, p-GSK-3β (Cell Signaling Technology, Beverly, MA), α-tubulin (Transduction Laboratories, Lexington, KY). Fluorescein- and rhodamine-tagged secondary antibodies (Roche, Indianapolis, IN) were used at a concentration of 1:250.
Luciferase Reporter Gene Assays
Luciferase reporter gene assays were conducted using the Luciferase Assay System (Promega, Madison, WI) and its corresponding protocol. All plasmids (500 ng) were transfected into cells using Lipofectamine and Plus reagents (Invitrogen) according to the manufacturer's guidelines. Light units were measured with a Luminometer TD-20/20 (Turner Designs, Mountain View, CA). Assays were normalized for transfection efficiency by cotransfecting cells with a β-gal control plasmid and were detected with the Luminescent β-gal control assay kit (Clontech, Palo Alto, CA). Experimental (luciferase) results were divided by the β-gal results to provide normalized data in arbitrary units. The pTOPFLASH-Lux and pFOPFLASH-Lux reporter constructs were generously provided by Dr. Hans Clevers (Netherlands Institute for Developmental Biology, Utrecht, The Netherlands). The pGL3-E-cad-Lux reporter construct was kindly provided by Dr. Shoukat Dedhar (University of British Columbia, Vancouver, Canada).
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
RNA extractions were performed using the RNeasy Mini kit (Qiagen, Chatsworth, CA) using 107 cells per sample. RT-PCR was conducted using the Super-ScriptII kit (Invitrogen) and corresponding protocol. Samples were incubated with a PTC-100 thermal cycler (MJ Research, Waltham, MA), with 30 cycles per sample. The following primer sequences were used: Snail: Forward: 5′-CCCAAGCCCAGCCGATGAG-3′; Reverse: 5′-CTTGGCCACGGAGAGCCC-3′; LEF-1: Forward: 5′-CTACCACGACAAGGCCAGAG-3′; Reverse: 5′-CAGTGAGGATGGGTAGGGTTG-3′; GAPDH: Forward: 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′; Reverse: 5′-CATGTAGGCCATGAGGTCCACCAC-3′. Samples were run on 1.5% agarose gels containing EtBr (1.5 μl) and visualized with a ChemiDoc XRS Imager (Biorad, Richmond, CA).
Cell Invasion Assays
Three-dimensional collagen gels (50% [RPMI + 1% penicillin/streptomycin] + 10% 10× F12, 10% sodium bicarbonate, 30% dialyzed collagen) were polymerized at 37°C in 96-well culture plates. MDCKII cells were then grown to near confluence on top of each gel, followed by experimental treatments. Assessment of cell invasion occurred by adjustment of the focal plane away from the monolayer into the gel, where individual invasive cells were counted.
RESULTS
Confirmation of Smad-dependent and Smad-independent TGF-β Signaling
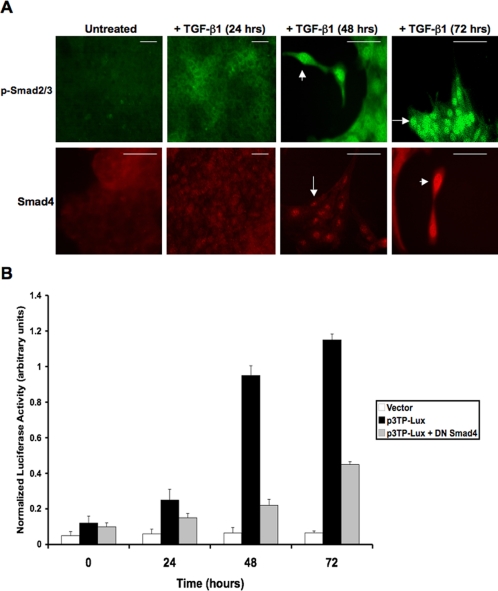
To demonstrate TGF-β signaling in MDCK cells, immunocytochemistry was performed using antibodies against phospho-Smad2/3 (p-Smad2/3) and Smad4. Cells were treated with TGF-β1 (10 ng/ml) for periods of 0 (untreated), 24, 48, and 72 h, with nuclear localization of Smads occurring at 48 h (Figure 1A; arrows). To assess Smad transcriptional activity, a p3TP-Lux reporter plasmid (possessing TGF-β response elements) was transfected into cells under the same conditions. Significant luciferase activity was detected at 48 h, correlating to the previously observed nuclear localization of Smad proteins. Introduction of a dominant negative (DN) Smad4 expression plasmid significantly reduced p3TP-Lux activity (Figure 1B). Smad-independent signaling pathways were confirmed as previously described (Peinado et al., 2003) via immunoblotting, demonstrating increased levels of both phospho-ERK1/2 (p-ERK1/2; Supplementary Figure S1A) and phospho-AKT (p-AKT; Supplementary Figure S1B) within 24 h of treatment with TGF-β1. By using a small molecule inhibitor against MEK1/2 (U0126), p-ERK1/2 expression was greatly reduced. Because appropriate separation of p-ERK1 and p-ERK2 bands was not achieved, we conducted densitometry (ImageJ) to further support these data. Mean density for untreated, TGF-β1-treated, and TGF-β1 + U0126-treated samples, respectively, were as follows: p-ERK1/2: 100.724, 152.897, 65.741; α-tubulin: 209.690, 207.689, 209.537. Similarly, a PI3K inhibitor (LY294002) significantly reduced phosphorylation of AKT. These results demonstrated that TGF-β1 signals through MEK and PI3K pathways (within 24 h), followed by the Smad pathway (within 48 h).
Figure 1.
TGF-β1 stimulates Smad, MEK, and PI3K pathways in MDCKII cells. (A) Immuocytochemistry demonstrated TGF-β1 (10 ng/ml) induced Smad signaling via nuclear translocation (arrows) of p-Smad2/3 and Smad4 within 48 h of treatment (scale bar, 20 μm). (B) Smad transcriptional activity was confirmed by a p3TP-Lux reporter gene construct, with significant luciferase activity correlating with the nuclear localized Smad proteins. Addition of a DN Smad4 construct significantly inhibited p3TP-Lux activity (p < 0.04).
TGFβ-1 Confers Loss of Tight Junction Protein Expression
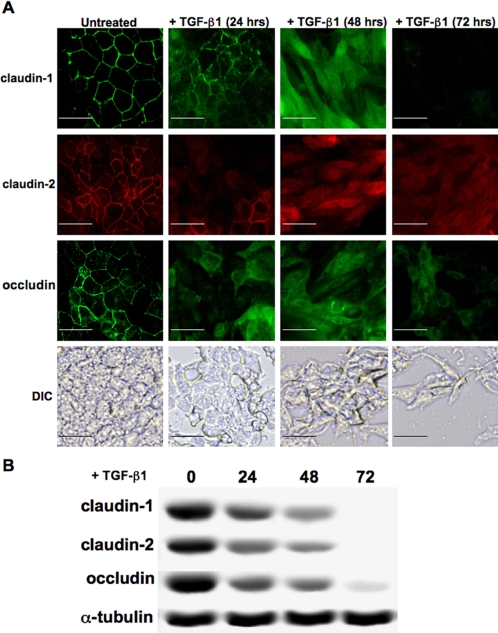
We next observed the effect of TGF-β1 on the expression of tight junctions proteins. Immunostaining for claudin-1, claudin-2, and occludin demonstrated that these proteins were fully removed from the cell junctions between 24 and 48 h of treatment with TGF-β1. A clear change in cellular morphology to fibroblastlike cells was also observed at 48 h, which was confirmed by DIC imaging (Figure 2A). Assessment of protein expression for claudin-1, claudin-2, and occludin was made via immunoblotting cell lysates from the same time points, showing continuous down-regulations of these proteins within 72 h (Figure 2B).
Figure 2.
TGF-β1 suppresses tight junction proteins and promotes a clear change in cell morphology. (A) Expression and localization of the tight junction proteins claudin-1, claudin-2, and occludin were assessed via immunostaining, demonstrating losses of these molecules in the cell membrane within 24 h of treatment with TGF-β1. The epithelial nature of these cells changed dramatically into fibroblastlike morphology within 48 h (scale bar, 20 μm). (B) Decreases in expression levels of claudin-1, claudin-2, and occludin were confirmed via Western blotting, with nearly complete loss at 72 h.
TGF-β1 Promotes Loss of Adherens Junctions and EMT
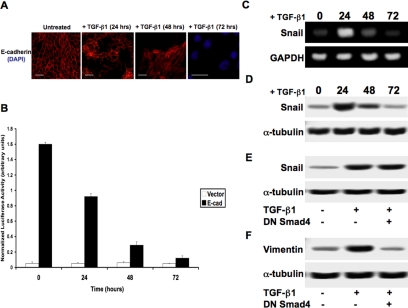
Based on the previously observed loss of tight junctions and the clear change in cell morphology, we confirmed loss of E-cadherin via immunostaining. Through microscopy we observed moderate breakage in cell-cell adhesion within the monolayer within 24 h of treatment with TGF-β1, with nearly all expression inhibited at 48 and 72 h as shown by immunofluorescence (Figure 3A). Next, we observed E-cadherin promoter activity by transfecting cells with a pGL3-E-cad-Lux reporter construct, which confirmed the results observed with our immunocytochemistry (Figure 3B). We then conducted RT-PCR to detect relative expression levels of the E-cadherin repressor Snail, thus confirming results previously published (Peinado et al., 2003) under the same conditions. We found that the peak of Snail expression occurs at 24 h after exposure to TGF-β1, followed by a steady decline in expression (Figure 3C). Protein expression of Snail appeared to be nearly identical to our RT-PCR results as observed via immunoblotting (Figure 3D). When comparing these results we found that ∼40-50% of E-cadherin promoter activity was reduced during the pinnacle of Snail expression (24 h). However, full repression of E-cadherin promoter activity was not achieved until 48-72 h, at which time Snail expression is barely detectable. We then showed that this increase in Snail expression occurred independently of Smad signaling 24 h after treatment with TGF-β1, as no effect on protein expression was observed in the presence of DN Smad4 (Figure 3E). To biochemically confirm that the TGF-β1 inhibition of E-cadherin associates with EMT, we performed immunostaining for the mesenchymal marker Vimentin, which showed greatly increased expression after treatment with TGF-β1 for 72 h (Supplementary Figure S2). These results were further confirmed by immunoblotting for Vimentin expression, showing that its increase is Smad-dependent as addition of DN Smad4 prevented its up-regulation (Figure 3F).
Figure 3.
TGF-β1 promotes an epithelial-mesenchymal transition. (A) Expression levels of E-cadherin were observed by immunocytochemistry in MDCKII cells exposed to TGF-β1. Most of the E-cadherin protein was repressed at 48 h, with no detection at 72 h (scale bar, 20 μm). (B) These results were confirmed using a pGL3-E-cad-Lux reporter gene construct, with ∼40-50% of E-cadherin promoter activity was reduced within 24 h of treatment, followed by nearly full repression at 48-72 h (p < 0.017). (C) RT-PCR analysis showed levels of the E-cadherin repressor Snail peak at 24 h posttreatment, with a steady decline in expression through 72 h. (D) Immunoblotting demonstrated that Snail protein expression followed the same pattern as mRNA expression. (E) Additional immunoblotting for Snail at 24 h posttreatment showed that DN Smad4 had no effect on expression levels, suggesting that this mechanism is Smad-independent. (F) To confirm EMT, expression of the mesenchymal marker Vimentin was observed via immunoblotting in cells treated with TGF-β1 for 72 h. This expression increase was found to be Smad-dependent, as DN Smad4 prevented increased levels of Vimentin (scale bar, 20 μm).
EMT Is Associated with Nuclear β-Catenin/LEF-1 Transcription Complexes
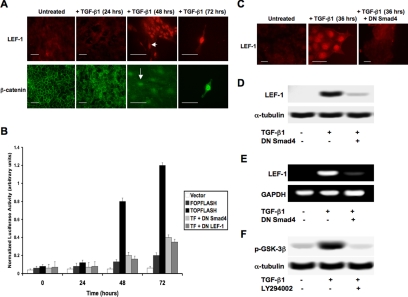
Because TGF-β has recently been linked to cross-talk mechanisms with traditional Wnt signaling molecules (Labbe et al., 2000; Nishita et al., 2000; Nawshad and Hay, 2003), we assessed the potential role of β-catenin/LEF-1 signaling, corresponding to the previously observed Smad signaling. Immunocytochemistry using antibodies against β-catenin and LEF-1 was conducted, showing nuclear localization of each at 48 h posttreatment with TGF-β1 (Figure 4A). LEF-1 transcriptional activity was observed using a pTOPFLASH-Lux reporter plasmid. Significant levels of luciferase activity were detected at 48 and 72 h, which directly correlated with the time points of nuclear localization. pFOPFLASH-Lux, a reporter construct with mutated LEF-1-binding sites, was used as a negative control. The addition of DN Smad4 or DN LEF-1 expression constructs greatly inhibited luciferase activity (Figure 4B). LEF-1 was not expressed in untreated MDCK cells, but 36 h after treatment with TGF-β1 we could detect LEF-1 expression via immunostaining. However, if cells were treated with DN Smad4, TGF-β1 could not induce LEF-1 expression, suggesting that this mechanism is Smad-dependent (Figure 4C). These results were further confirmed by immunoblotting (Figure 4D) and RT-PCR (Figure 4E). Because the formation of β-catenin/LEF-1 complexes is unlikely without inhibition of GSK-3β, we conducted immuno-blotting analysis for GSK-3β phosphorylation. Within 24 h of exposure to TGF-β1, significant increases in p-GSK-3β were observed. Because Wnt signaling was highly unlikely to occur in culture and molecules downstream of PI3K (such as AKT) have been known to phosphorylate GSK-3β, we performed the same experiment in the presence of a PI3K inhibitor (LY294002). Our results demonstrated that inhibition of PI3K dramatically reduced levels of p-GSK-3β (Figure 4F).
Figure 4.
β-Catenin/LEF-1 signaling correlates with the transition to mesenchyme. (A) Nuclear localization (arrows) of β-catenin and LEF-1 were observed via immunostaining within 48 h of treatment with TGF-β1 (scale bar, 20 μm). (B) LEF-1 transcriptional activity was shown to correspond to the previously observed nuclear localization (A) using a pTOPFLASH-Lux reporter gene construct, with pFOPFLASH-Lux used as a negative control. DN Smad4 and DN LEF-1 significantly inhibited pTOPFLASH luciferase activity (p < 0.024). (C) The TGF-β1-induced LEF-1 expression observed via immunostaining was Smad-dependent, as we showed that expression was inhibited in cells containing a DN Smad4 construct (scale bar, 20 μm). These results were further confirmed by conducting (D) immunoblotting and (E) RT-PCR under the same conditions. (F) TGF-β1 was also shown to assist formation of the β-catenin/LEF-1 transcription complexes by preventing degradation of cytoplasmic β-catenin. We observed via immunoblotting that TGF-β1 promotes phosphorylation of GSK-3β within 24 h. This up-regulation of p-GSK-3β in inhibited in cells treated with a PI3K inhibitor, suggesting that this pathway is responsible for the associated phosphorylation.
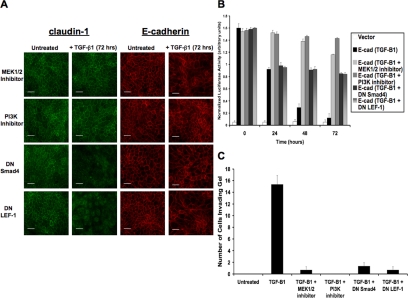
MEK and PI3K Pathways Promote Tight Junction Loss, But EMT Is Dependent on Smad and β-Catenin/LEF-1 Pathways
Immunocytochemistry was performed for detection of claudin-1 and E-cadherin upon stimulation with TGF-β1 (72 h) in the presence of MEK1/2 inhibitor (U0126), PI3K inhibitor (LY294002), DN Smad4, or DN LEF-1 (Figure 5A). We found that claudin-1 remained expressed in the membranes of cells exposed to MEK and PI3K inhibitors, whereas DN Smad4 and DN LEF-1 did not prevent loss of claudin-1. However, we observed that all four inhibitors were sufficient to prevent full loss of E-cadherin within the cell junctions, indicating that Smad4 and LEF-1 are necessary for the loss of adherens junctions and the transition to mesenchyme. E-cadherin promoter activity (pGL3-E-cad-Lux) was detected over time periods of 0, 24, 48, and 72 h of stimulation with TGF-β1 in the presence of the same inhibitors (Figure 5B). We determined that MEK and PI3K inhibitors retained nearly all of the promoter activity, but DN Smad4 and DN LEF-1 only retained 50-60%. These findings correlated with the earlier results that demonstrated a 40-50% loss of E-cadherin during the peak of Snail expression (24 h). These results show that MEK and PI3K pathways, which have previously been proven to up-regulate Snail expression (Peinado et al., 2003), are responsible for suppressing approximately half of the total cellular E-cadherin. However, full E-cadherin repression was associated with our findings of nuclear localization and transcriptional activities of Smad4 and LEF-1, suggesting that both Snail and LEF-1 are necessary for complete loss of E-cadherin and completion of EMT. We then assessed TGF-β1-induced cell invasion into three-dimensional collagen gels using the same inhibitors (Figure 5C). We showed that TGF-β1 stimulates high levels of invasion, whereas treatment with MEK1/2 inhibitor, PI3K inhibitor, DN Smad4, or DN LEF-1 dramatically reduces cell invasion. These results confirm that both Smad-dependent and Smad-independent signaling pathways are necessary for EMT.
Figure 5.
MEK and PI3K pathways suppress tight junctions, but full repression of adherens junctions and stimulation of EMT are dependent on Smad and β-catenin/LEF-1 signaling. (A) Expression of claudin-1 and E-cadherin was observed via immunocytochemistry in the presence of various inhibitors to assess the signaling pathways that control loss of tight junctions and adherens junctions during TGF-β-induced EMT. MEK1/2 and PI3K inhibitors prevented loss of claudin-1, whereas DN Smad4 and DN LEF-1 constructs did not. However, all inhibitors prevented loss of E-cadherin, suggesting that cooperation between all of these pathways is responsible for loss of adherens junctions and the transformation to mesenchyme (scale bar, 20 μm). (B) E-cadherin promoter activity was measured using the pGL3-E-cad reporter gene construct under the same conditions and showed that MEK and PI3K pathways correlate with expression of Snail. The later pathways of Smad and β-catenin/LEF-1 confer full repression of E-cadherin (p < 0.012). (C) Cell invasion into collagen gels was assessed showing high levels of invasion upon treatment with TGF-β1. The addition of MEK1/2 inhibitor, PI3K inhibitor, DNSmad4, or DN LEF-1 greatly decreased levels of cell invasion.
DISCUSSION
The work of Peinado et al. (2003) clearly established a necessary role of Snail in mediating TGF-β1-induced EMT in MDCK cells, by demonstrating that the Ras-Raf-MEK-ERK-AP-1 signaling pathway could up-regulate synthesis of the E-cadherin repressor molecule Snail. Taken alone, these data suggest the transformation of MDCK cells to mesenchyme occurred by a Smad-independent mechanism. However, recent findings of the importance of Smad-dependent signaling during EMT and its link to LEF-1 up-regulation (Nawshad and Hay, 2003) persuaded us to assess any potential role for Smad or β-catenin/LEF-1 in TGF-β1-mediated EMT.
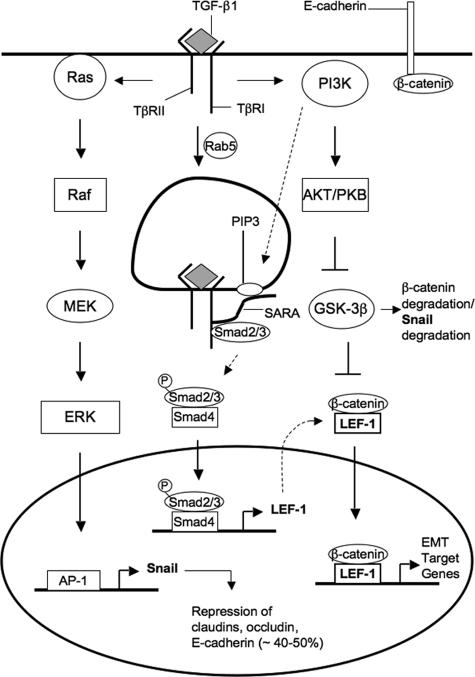
From our results we established that both Smad-dependent and Smad-independent signaling were necessary to drive EMT. The previously described TGF-β signaling via Ras-Raf-MEK-ERK-AP-1, with peak expression of Snail at 24 h posttreatment, is clearly responsible for dissociation of tight junctions and a partial loss of E-cadherin. PI3K is a necessary element for allowing proper function of Snail, as its downstream effectors like AKT can phosphorylate (and thus deactivate) GSK-3β, a molecule that promotes degradation of Snail (Zhou et al., 2004) and β-catenin (Waterman, 2004). However, PI3K is also a required mediator of Smad signaling. In the early endosome, TβRI (ALK5) phosphorylates Smad proteins upon their recruitment by SARA. However, for proper association with the receptor, SARA must also bind with PIP3 (which is formed by PI3K) in the endosomal membrane through its zinc-finger FYVE domain (Itoh et al., 2002). We established that upon Smad phosphorylation and translocation to the nucleus, this Smad2/3-Smad4 transcription complex would induce expression of LEF-1. Because GSK-3β was inactivated, cytoplasmic pools of β-catenin would then associate with LEF-1 to form transcription complexes that would target genes necessary for EMT, including full repression of E-cadherin (Figure 6).
Figure 6.
A schematic representation of the proposed TGF-β1 signaling mechanism that promotes EMT. Early signaling through Ras-Raf-MEK-ERK-AP-1 causes up-regulation in Snail expression. Snail, a well-known repressor of junction proteins, then inhibits expression of claudin-1, claudin-2, and occludin. Snail also provides partial loss of E-cadherin, thus decreasing the level of substrate for β-catenin. Further stabilization of cytoplasmic β-catenin is achieved through PI3K signaling. Molecules downstream of PI3K such as AKT phosphorylate and deactivate p-GSK-3β, which is responsible for degrading both β-catenin and Snail through the ubiquitin proteosome pathway. Smad signaling, controlled through endocytosis of TGF-β receptor complex, promotes transcription of LEF-1, an alternative substrate for β-catenin. On forming β-catenin/LEF-1 complexes, these molecules will then act to transcribe genes that will induce EMT.
Our results provide the first model to suggest that LEF-1 and Snail are both necessary for stimulation of EMT. Both transcription factors are expressed in tissues that undergo EMT during embryogenesis (Nawshad and Hay, 2003; Mohamed et al., 2004; Vega et al., 2004) and have been described to promote the invasive phenotype in cancer cells (Cano et al., 2000; Kim et al., 2002). However, until now, a link between these transcription factors has not been made. Several experiments have been performed to assess whether β-catenin/LEF-1 can up-regulate Snail to induce EMT, but no such results were established (Kim et al., 2002; Peinado et al., 2003). We now see that TGF-β1 initiates the function of these transcription factors through vastly different, though interconnecting, signaling pathways.
Although most of our experiments were conducted under the same conditions, our findings were somewhat different from those of Peinado et al. (2003). It was previously published that although TGF-β1 appeared to induce EMT, protein expression levels of the epithelial marker E-cadherin remained high at 72 h posttreatment with TGF-β1. Also, no significant increase of Vimentin, (perhaps the only true mesenchymal marker) was observed, although formation of intermediate filaments correlated with the mesenchymal phenotype. These results did not present a definitive demonstration that EMT is occurring. However, our findings conclude that there is indeed a complete repression of E-cadherin, as observed via immunocytochemistry and reporter gene analysis of E-cadherin promoter activity. Also, our immunostaining for Vimentin showed a significant increase in its expression 72 h posttreatment with TGF-β1. These data biochemically support that TGF-β1 promoted EMT in MDCK cells.
Although we have now established a mechanism that unifies many of the key molecules that have been associated with EMT, other signaling pathways have been described to induce EMT. Recent evidence suggests that NF-κB signaling plays an essential role in this transition (Huber et al., 2004) and may influence Snail expression (Bachelder et al., 2005). Snail up-regulation has also recently been linked to the MMP-3-Rac1-ROS pathway to help drive EMT (Radisky et al., 2005). Other pathways such as Notch (Zavadil et al., 2004) and RhoA (Bhowmick et al., 2001) have also been implicated in EMT, although it is not yet clear as to how they can be integrated into the mechanism we describe here. Although we have successfully demonstrated a synergy between four major signaling pathways to stimulate EMT, we must consider that this cascade may be cell-type dependent.
Observing this signaling mechanism in MDCK cells may provide certain clinical applications. EMT, although predominantly a developmental mechanism, is also a potent initiator of pathological conditions such as tumor metastasis (Thiery, 2002). In kidney cells, this mechanism is responsible for induction of renal fibrosis. TGF-β1 has previously been linked to this disorder, demonstrating that when the downstream signaling of TGF-β is counteracted by BMP7, EMT is reversed to restore proper function of the organ (Kalluri and Neilson, 2003; Zeisberg et al., 2003).
TGF-β signaling pathways are extensive and complex in nature. The in vitro environment used for these experiments (isolating only TGF-β signaling) are significantly different from that which occurs in vivo. We must also consider that these results are cell-type dependent and would need to be confirmed in other systems. However, the unified signaling mechanism that we describe with this work should contribute to a better understanding of the molecular mechanisms of EMT.
Supplementary Material
Acknowledgments
We thank Dr. Malcolm Whitman (Department of Developmental Biology: Harvard School of Dental Medicine) for his careful reading of our manuscript and helpful advice. This work was supported by DE11142 (E.D.H.) and GM18974 (D.A.G.) from the U.S. Department of Public Health.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0767) on February 8, 2006.
Abbreviation used: DN, dominant negative; EMT, epithelial-mesenchymal transition; ERK, extracellular signal-related kinase; GSK-3β, glycogen synthase kinase-3 beta; ILK, integrin linked kinase; LEF-1, lymphoid enhancer factor-1; PI3K, phosphoinositide-3-kinase; TβR, transforming growth factor-beta receptor; TGF-β, transforming growth factor-beta.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Akhurst, R. J., and Derynck, R. (2001). TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 11, S44-S51. [DOI] [PubMed] [Google Scholar]
- Bachelder, R. E., Yoon, S. O., Fanci, C., de Herreros, A. G., and Mercurio, A. M. (2005). Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J. Cell Biol. 168, 29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick, N. A., Ghiassi, M., Bakin, A., Aakre, M., Lundquist, C. A., Engel, M. E., Artega, C. L., and Moses, H. L. (2001). Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 12, 27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano, A., Perez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., del Barrio, M. G., Portillo, F., and Nieto, M. A. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76-83. [DOI] [PubMed] [Google Scholar]
- Galceran, J., Sustmann, C., Hsu, S. C., Folberth, S., and Grosschedl, R. (2004). LEF-1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev. 18, 2718-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro, M., and Bronner-Fraser, M. (1999). Induction and differentiation of the neural crest. Curr. Opin. Cell Biol. 11, 695-698. [DOI] [PubMed] [Google Scholar]
- Hay, E. D. (1995). An overview of epithelio-mesenchymal transformation. Acta Anat. 154, 8-20. [DOI] [PubMed] [Google Scholar]
- Hannigan, G., Troussard, A. A., and Dedhar, S. (2005). Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat. Rev. Cancer 5, 51-63. [DOI] [PubMed] [Google Scholar]
- Huber, M. A., Azoitei, N., Baumann, B., Grunert, S., Sommer, A., Pehamberger, H., Krauta, N., Beug, H., and Wirth, T. (2004). NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi, J., Matsuda, M., Furuse, M., and Tsukita, S. (2003). Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occluding by Snail. J. Cell Sci. 116, 1959-1967. [DOI] [PubMed] [Google Scholar]
- Itoh, F., Divecha, N., Brocks, L., Oomen, L., Janssen, H., Calafat, J., Itoh, S., and ten Dijke, P. (2002). The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signaling. Genes Cells 7, 321-331. [DOI] [PubMed] [Google Scholar]
- Jamora, C., DasGupta, R., Kocieniewski, P., and Fuchs, E. (2003). Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422, 317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda, E., Lehmann, K., Killisch, I., Jechlinger, M., Herzig, M., Downward, J., Beug, H., and Grunert, S. (2002). Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri, R., and Neilson, E. G. (2003). Epithelial-mesenchymal transition and its implication for fibrosis. J. Clin. Invest. 112, 1776-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K., Lu, Z., and Hay, E. D. (2002). Direct evidence for a role of betacatenin/LEF-1 signalling pathway in the induction of EMT. Cell Biol. Int. 26, 463-476. [DOI] [PubMed] [Google Scholar]
- Labbe, E., Letamendia, A., and Attisano, L. (2000). Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc. Natl. Acad. Sci. USA 97, 8358-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Murai, Y., Okada, E., Matsui, K., Hayashi, S., Horie, M., and Takano, Y. (2002). Modified and simplified western blotting protocol: use of intermittent irradiation (IMWI) and 5% skim milk to improve binding specificity. Pathol. Int. 52, 234-238. [DOI] [PubMed] [Google Scholar]
- Massague, J. (2004). G1 cell-cycle control and cancer. Nature 432, 298-306. [DOI] [PubMed] [Google Scholar]
- Mohamed, O. A., Clarke, H. J., and Dufort, D. (2004). Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev. Dyn. 231, 416-424. [DOI] [PubMed] [Google Scholar]
- Nawshad, A., LaGamba, D., Polad, A., and Hay, E. D. (2005). Transforming growth factor beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tiss. Org. 179, 11-23. [DOI] [PubMed] [Google Scholar]
- Nawshad, A., and Hay, E. D. (2003). TGFβ3 signaling activates transcription of the LEF1 gene to induce epithelial-mesenchymal transformation during mouse palate development. 163, 1291-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita, M., Hashimoto, M. K., Ogata, S., Laurent, M. N., Ueno, N., Shibuya, H., and Cho, K. W. (2000). Interaction between Wnt and TGF-beta signaling pathways during formation of Spemann's organizer. Nature 403, 781-785. [DOI] [PubMed] [Google Scholar]
- Ohkubo, T., and Ozawa, M. (2004). The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J. Cell Sci. 117, 1675-1685. [DOI] [PubMed] [Google Scholar]
- Ozdamar, B., Bose, R., Barrios-Rodiles, M., Wang, H. R., Zhang, Y., and Wrana, J. L. (2005). Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307, 1603-1609. [DOI] [PubMed] [Google Scholar]
- Peinado, H., Portillo, F., and Cano, A. (2004). Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 48, 365-375. [DOI] [PubMed] [Google Scholar]
- Peinado, H., Quintanilla, M., and Cano, A. (2003). Transforming growth factor beta-1 induces Snail transcription factor in epithelial cell lines: implications for epithelial mesenchymal transitions. J. Biol. Chem. 23, 21113-21123. [DOI] [PubMed] [Google Scholar]
- Radisky, D. C. et al. (2005). Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., and Massague, J. (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685-700. [DOI] [PubMed] [Google Scholar]
- ten Dijke, P., and Hill, C. S. (2004). New insights into TGF-beta-Smad signaling. Trends Biochem. Sci. 29, 265-273. [DOI] [PubMed] [Google Scholar]
- Thiery, J. P. (2002). Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer 2, 442-454. [DOI] [PubMed] [Google Scholar]
- Vega, S., Morales, A. V., Ocana, O. H., Valdes, F., Fabregat, I., and Nieto, M. A. (2004). Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 18, 1131-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman, M. L. (2004). Lymphoid enhancer factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 23, 41-52. [DOI] [PubMed] [Google Scholar]
- Xie, L., Law, B. K., Chytil, A. M., Brown, K. A., Aakre, M. E., and Moses, H. L. (2004). Activation of the Erk pathway is required for the TGF-beta1-induced EMT in vitro. Neoplasia 6, 603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Mani, S. A., Donaher, J. L., Ramaswamy, S., Itzykson, R. A., Come, C., Savagner, P., Gitelman, I., Richardson, A., and Weinberg, R. A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927-939. [DOI] [PubMed]
- Yi, J. Y., Shin, I., and Artega, C. L. (2005). Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J. Biol. Chem. 280, 10870-10876. [DOI] [PubMed] [Google Scholar]
- Zavadil, J., Cermak, L., Soto-Nieves, N., and Bottinger, E. P. (2004). Integration of TGF-beta/Smad and Jagged1/Notch signaling in epithelial-to-mesenchymal transition. EMBO J. 23, 1155-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg, M., and Kalluri, R. (2004). The role of epithelial-to-mesenchymal transition in renal fibrosis. J. Mol. Med. 82, 175-181. [DOI] [PubMed] [Google Scholar]
- Zeisberg, M., Hanai, J., Sugimoto, H., Mammoto, T., Charytan, D., Strutz, F., and Kalluri, R. (2003). BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964-968. [DOI] [PubMed] [Google Scholar]
- Zhou, B. P., Deng, J., Xia, W., Xu, J., Li, Y. M., Gunduz, M., and Hung, M. C. (2004). Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6, 931-940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.